Workshop on stains and staining
Saturday 6th April 2024
The workshop in the Angela Marmont Centre in the Natural History Museum got off to an unusual start. Most of the stains that we would be using only need to be applied for a short period, but haematoxylin needs to be applied for at least an hour. Terry Hope provided slides with sections of rat stomach, so we started by either dipping the slides in haematoxylin, or applying a drop with a pipette.
 Stains and equipment
Stains and equipment
 Nigel Williams applying haematoxylin
Nigel Williams applying haematoxylin
While the staining was left to work, Terry gave a talk on the history and uses of stains and staining.
 Terry Hope’s introductory talk
Terry Hope’s introductory talk
Pollen
Terry brought a bag of flowers from his garden so that we could make slides of pollen. The mountant was glycerine jelly that contains basic fuchsin, and is easy to use. Basic fuchsin is a magenta stain. The jelly needs to be warm, so the jar was kept in a mug of warm water until it was needed. We used a small brush to transfer pollen onto a slide, then a glass rod to apply a drop of the jelly, and finally added a coverslip. The pollen gradually takes up the fuchsin from the jelly. Glycerine jelly does not set hard, and it is hygroscopic, so to make slides permanent they need to be ringed with nail varnish, shellac, or a commercial ringing cement.
 Transferring pollen to a slide
Transferring pollen to a slide
 Adding glycerine jelly (with basic fuchsin) to pollen slide
Adding glycerine jelly (with basic fuchsin) to pollen slide
Cheek cells
We were provided with smooth wooden spatulas for gently scraping the insides of our cheeks and then transferring the material to a slide. We dried the smears on the makeshift hotplate, then used a pipette to add a little methylene blue. Methylene blue is a blue stain that makes cell nuclei more visible. The methylene blue was left for about 2 minutes, and then washed off with water.
 David and Rhodri Lewis adding methylene blue to cheek smear
David and Rhodri Lewis adding methylene blue to cheek smear
The Quekett members who had intended to bring hotplates were unable to attend because of the train strikes, so Lisa Ashby improvised with two hot-water bottles and a thin metal sheet.
 Makeshift hotplate
Makeshift hotplate
Blood smears
Terry Hope pricked his thumb using a lancet to produce drops of blood. He showed us how to transfer a drop to the end of a slide, and then use a second slide to spread out the blood into a thin smear. We dried the slides, added some Giemsa stain, left it for 10–20 seconds, rinsed with alcohol, and then dried again. Giemsa stains red blood cells a different colour from white blood cells and platelets.
Rat stomach sections
Finally we returned to the sections of rat stomach that had been staining in haematoxylin since the start of the workshop. We rinsed off the excess stain with acid alcohol. Haematoxylin is red but can be turned to blue with alkaline, so we then rinsed the slides with an alkaline solution.
 Bluing haematoxylin with alkaline rinse
Bluing haematoxylin with alkaline rinse
We then applied a second stain, eosin, and rinsed it off after a few seconds. The blued haematoxylin stains the nuclei of cells a bluish colour, and the eosin stains cytoplasm a pinkish colour. The combination is often referred to as H&E stain.
 Drying slides
Drying slides
We were then able to examine the slides under a microscope, with a 40× objective giving the best view.
 Examining the slides
Examining the slides
 Examining the slides
Examining the slides
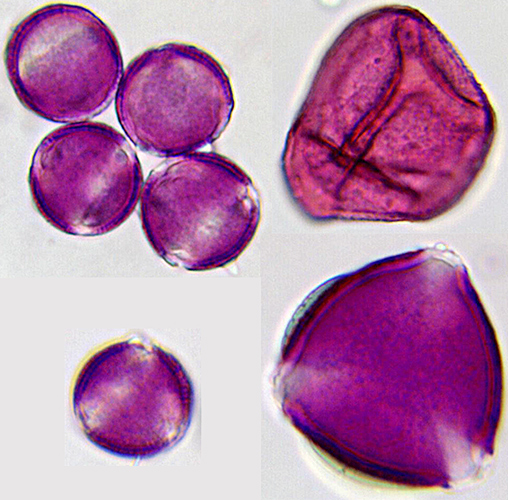 Pollen grains stained with basic fuchsin (20× objective) [photomicrographs by Pam Hamer]
Pollen grains stained with basic fuchsin (20× objective) [photomicrographs by Pam Hamer]
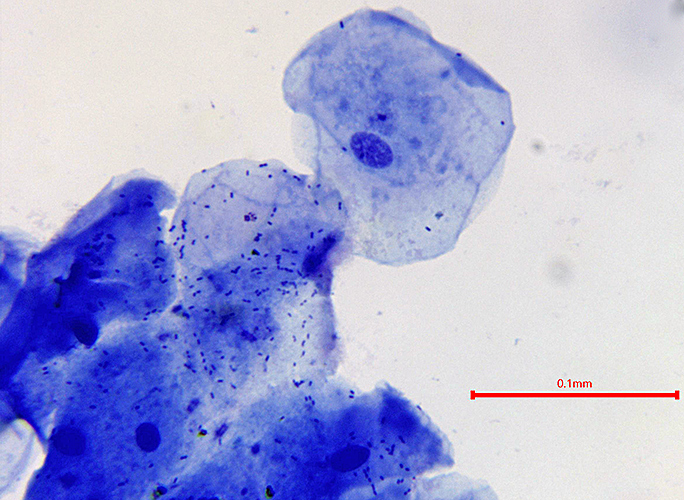 Cheek cells stained with methylene blue (20× objective) [photomicrograph by Pam Hamer]
Cheek cells stained with methylene blue (20× objective) [photomicrograph by Pam Hamer]
 Discussing the techniques
Discussing the techniques
 Stained slides and notes
Stained slides and notes
Gossip
Some Quekett members brought gossip exhibits.
Lisa Ashby brought some slides of stained specimens from her collection
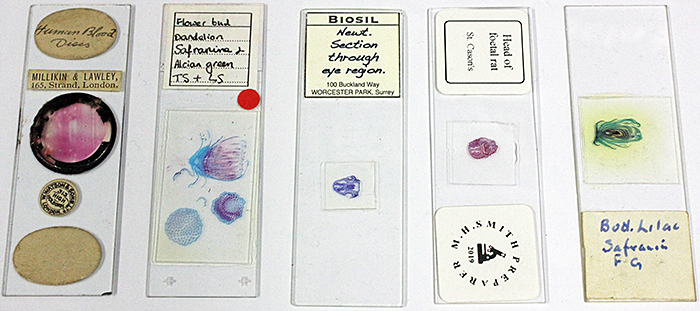 Prepared stained slides
Prepared stained slides
Lisa also brought some bits of an old pollen filter from her car, so that we could try to transfer the pollen to slides.
Nigel Ashby brought a copy of A History of Microtechnique by Brian Bracegirdle which contains an illustration of an apparatus for keeping embedding wax liquid, together with the version that he had constructed. A tin containing the wax sits on the wide end of a long, thin triangle of copper that is suspended above a baseboard. A spirit lamp placed below the narrow end provides the right amount of heat.
 Wax warmer
Wax warmer
Nigel Williams brought an arrangement of butterfly scales by Klaus Kemp, and we were able to admire it using a stereomicroscope.
 Nigel Williams’ exhibit
Nigel Williams’ exhibit
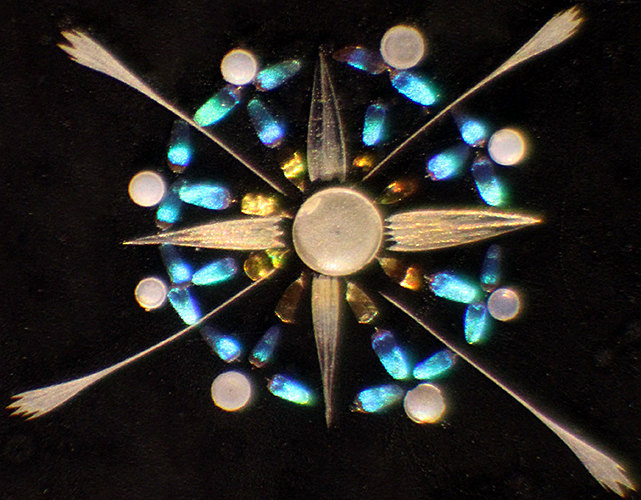 Butterfly scale star by Klaus Kemp [photomicrograph by Nigel Williams]
Butterfly scale star by Klaus Kemp [photomicrograph by Nigel Williams]
Pam Hamer showed some examples of staining non-biological material
Keith Abineri used to make peels of polished and etched rocks , and then stain the peels to help with identifying the minerals.
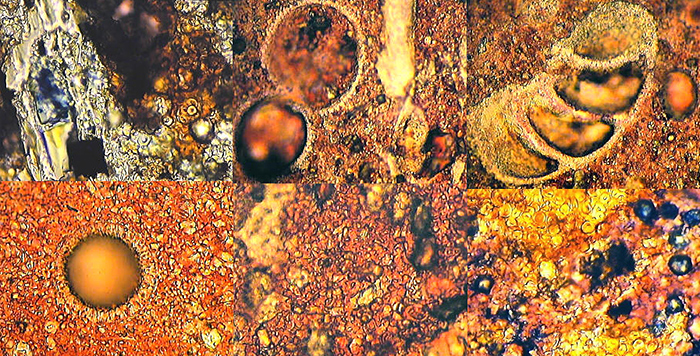 Stained rock peels
Stained rock peels
- Abineri, Keith W., The Preparation of Cellulose Lacquer Rock Peels, Quekett Journal of Microscopy 35, July–December 1986, 451–459
- Abineri, Keith W., Staining and Other Methods for Enhancing the Observation of Cellulose Lacquer Rock Peels, Micscape March 1999
- Abineri, Keith W., Microfossil and Nannofossil Image Gallery, Micscape October 1999
Staining can also be used to monitor microplastics. There are two recent paper on monitoring microplastics in water and sediment using Nile Red staining to produce fluorescence in the microplastics.
- Sturm, Michael Toni et al., Development of an Inexpensive and Comparable Microplastic Detection Method Using Fluorescent Staining with Novel Nile Red Derivatives, Analytica 2023, 4(1), 27–44
- Majcen, Alina et al., Lighting Up for Learning—Fluorescence Analysis of Microplastic Particles by Secondary School Students Using Nile Red Journal of Chemical Education 2023, 100(10), 4007–4012
 Nile Red for detecting microplastics
Nile Red for detecting microplastics
Report and most photographs by Alan Wood

