Webmaster’s blog 2020
Alan Wood’s microscopical diary
December 2020
A few months ago, a former Olympus employee in Germany who is a member of the Olympus Microscope Users Group on Facebook offered a lot of Olympus documents anyone who wanted them. I agreed to take them, and a 10 Kg parcel soon arrived with 2 lever-arch files bulging with Technical/Marketing Information sheets in English and German.
 Olympus Technical/Marketing Information
Olympus Technical/Marketing Information
They are almost all about the recent infinity-corrected microscopes, so I did not scan them, I just prepared indexes so that anyone interested can request a PDF.
November 2020
November brought another Zoom gossip meeting, this time on “Micro fossils”. I wasn’t sure if I had any suitable slides to show, but I discovered a slide of radiolarian fossil earth from Barbados (made by Brian Darnton), got out my Olympus BH2-DCD dark-ground condenser and SPlanApo 10× objective and took this photo with my Canon EOS 5D Mark II and EOS Utility. Seventy images at 0.002 mm intervals combined in Zerene Stacker. Levels adjusted and sharpened in Photoshop Elements 11.
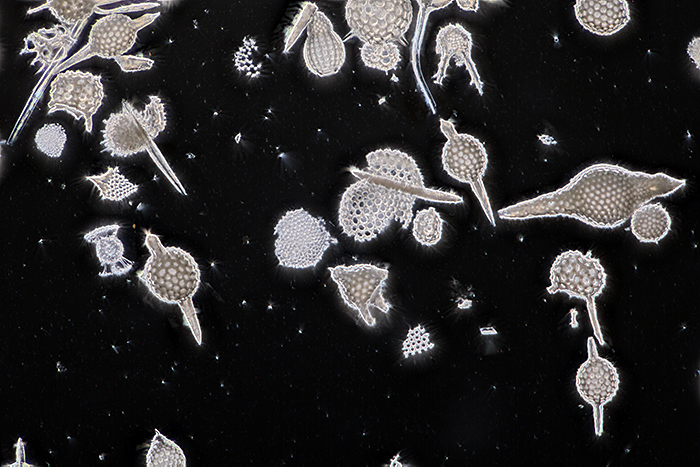 Radiolarian fossil earth from Barbados
Radiolarian fossil earth from Barbados
October 2020
I just about got everything ready for the virtual Annual Exhibition of Microscopy. We decided to make the exhibits, the photomicrographs, the slides and the artwork available on separate days. I used a password to prevent members seeing the pages in advance, and then rushed to remove the passwords at the appointed dates and times. It all worked pretty well, and making everything visible in the public area of the website encouraged non-members to have a look.
September 2020
When it became clear that it would not be possible to hold Quekex in the Natural History Museum this year, the committee decided to hold a virtual event spread over 6 days. Trying to hold everything on one day would have involved members staring at their computers for several hours, not good for their health or domestic harmony.
I normally take photographs and make notes at Quekex so that I can prepare a meeting report for the website, but with a virtual event I had to have everything prepared in advance. So I am back to working on the website instead of using a microscope. I had to convert all of the exhibits (supplied as Word, PDF and text files with lots of images) into web pages, prepare image galleries of all of the photomicrographs and slides submitted for Barnard Awards, and prepare pages to display the artwork and videomicrographs, all in time for the opening of the Exhibition.
For a while, it looked as though we were going to be short of photomicrographs submitted for Barnard Awards. I did not have time for any new shots, so I looked out some old ones and sent them off to Mike Gibson:
 Slides submitted for Barnard Awards
Slides submitted for Barnard Awards
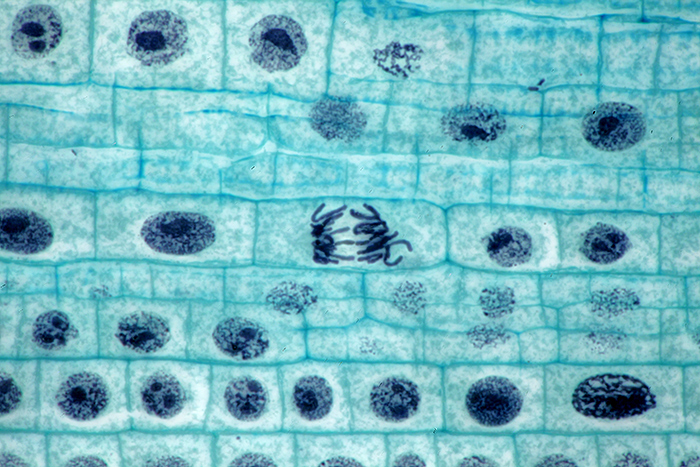 Allium l.s. root tip mitosis
Allium l.s. root tip mitosis
Olympus BHT with BH2-AAC condenser, SPlan 100× NA 0.95 dry objective and NFK 2.5× photo eyepiece. Canon EOS 5D Mark II camera. Stack of 8 images in Zerene Stacker.

Longitudinal section of an entire rabbit embryo, slide by Flatters & Garnett
Canon EOS 600D, 60 mm EF/S macro lens at f/13, illuminated by a light box
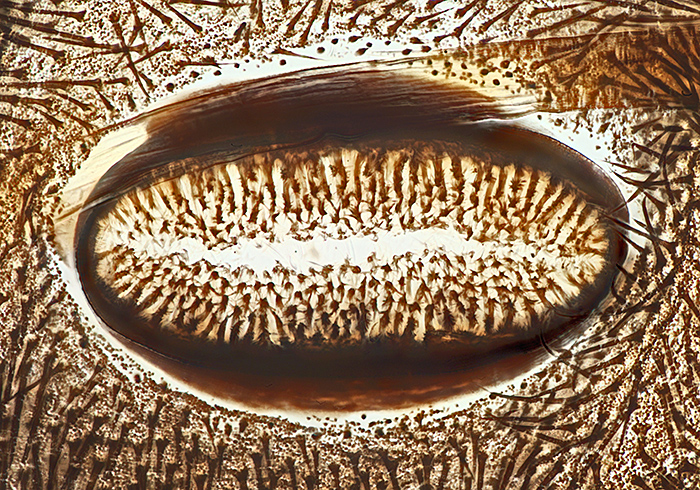
Spiracle of caterpillar of cinnabar moth (Tyria jacobaeae (L.)), slide by Eric Impey
Nikon NA 0.85 achromatic condenser, Olympus SPlan 40× objective, NFK 2.5× photo eyepiece, stack of 25 images at 2 µm intervals in Zerene Stacker
August 2020
Another job that I have been meaning to do for ages is calibrating an eyepiece reticle for my SPlan and MSPlan objectives (the ones that I normally use for photomicrographs) using my stage micrometers. I have an Olympus Micro WHK 10x/20 L eyepiece with a built-in 10 mm reticle that is divided into 100 parts of 0.1 mm; the top section can be rotated to focus on the reticle. The reticle is cracked, but the markings are not affected.
 Reticle in Olympus Micro WHK 10× eyepiece (photographed on a light box, with the top section of the eyepiece removed, Canon EOS 600D with 60 mm f/2.8 EF-S macro lens)
Reticle in Olympus Micro WHK 10× eyepiece (photographed on a light box, with the top section of the eyepiece removed, Canon EOS 600D with 60 mm f/2.8 EF-S macro lens)
I also have an assortment of old and recent 3″ × 1″ stage micrometers, including one for reflected light. I find the ones with engraved markings made by Graticules Optics (formerly Graticules Ltd) much easier to use than the modern Chinese ones with printed markings.
 Stage micrometers (top for transmitted light with a scale on clear glass, bottom for reflected light with a scale on a mirror)
Stage micrometers (top for transmitted light with a scale on clear glass, bottom for reflected light with a scale on a mirror)
To calibrate, focus on the stage micrometer, adjust the lighting for Köhler illumination, and use the focusing helix on the eyepiece to get the reticle in focus. Use the mechanical stage to line up the zero points of the stage micrometer and the eyepiece reticle. Find a point where the 2 scales line up nicely; in the photo below, 60 reticle divisions equal 3 micrometer divisions, which we know are 0.1 mm apart. Then a bit of arithmetic:
(3 × 0.1 ) ÷ 60 = 0.005 mm
tells us that 1 eyepiece reticle division equals 0.005 mm for this objective.
 Calibrating a 20× objective (thick lines are 0.1 mm apart on the stage micrometer, thin lines are the eyepiece reticle) (afocal setup, Canon EOS 5D Mark II with 50 mm f/1.8 EF standard lens supported on a copy stand above Micro WHK eyepiece in the vertical tube of a trinocular head)
Calibrating a 20× objective (thick lines are 0.1 mm apart on the stage micrometer, thin lines are the eyepiece reticle) (afocal setup, Canon EOS 5D Mark II with 50 mm f/1.8 EF standard lens supported on a copy stand above Micro WHK eyepiece in the vertical tube of a trinocular head)
I worked out the real sizes for 1, 10 and 100 reticle divisions for the objectives that I use most often:
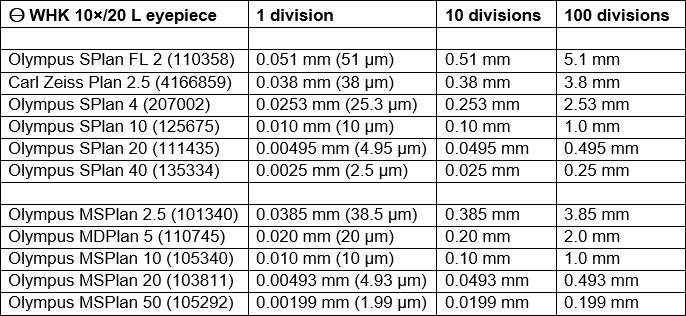 Eyepiece reticle calibrations
Eyepiece reticle calibrations
For the August gossip on Ernie Ives’ slides, I showed photos and photomicrographs of two of his wood section slides and two of his insect slides. Ernie used a consistent layout for the 3 wood sections on his slides; the large section on the left is transverse, top right is radial and bottom right is tangential.
 Wood section slides by Ernie Ives
Wood section slides by Ernie Ives
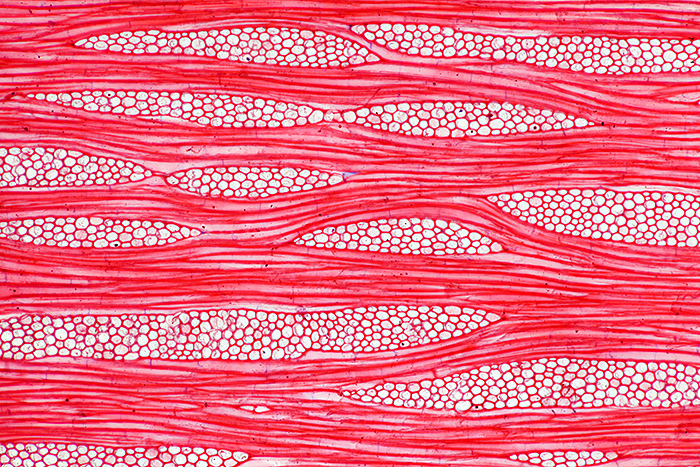 Tangential section of wood of teak (Tectona grandis), stained with safranin (10× objective, 2.5× photo eyepiece, Canon EOS 5D Mark II, EOS Utility)
Tangential section of wood of teak (Tectona grandis), stained with safranin (10× objective, 2.5× photo eyepiece, Canon EOS 5D Mark II, EOS Utility)
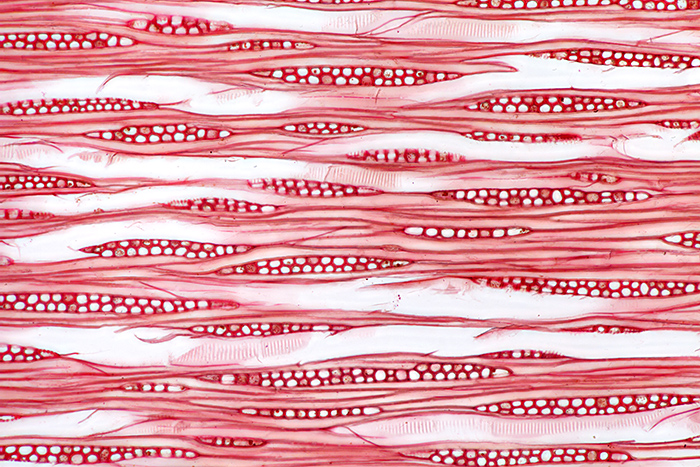 Tangential section of wood of southern magnolia (Magnolia grandiflora), stained with safranin (10× objective, 2.5× photo eyepiece, Canon EOS 5D Mark II, EOS Utility)
Tangential section of wood of southern magnolia (Magnolia grandiflora), stained with safranin (10× objective, 2.5× photo eyepiece, Canon EOS 5D Mark II, EOS Utility)
When I examined and photographed the insect slides for the gossip I was surprised that they were not up to Ernie’s usual high standard; one has lots of air bubbles, and the other has a broken specimen and very obvious supports for the coverslip that protect the specimen from being squashed..
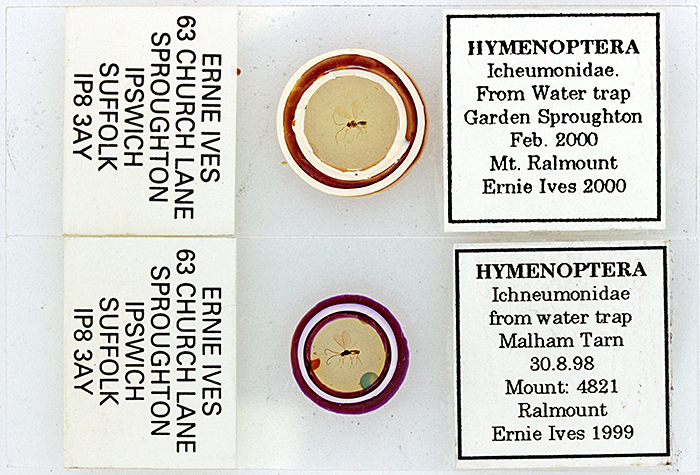 Slides of ichneumonids by Ernie Ives (Canon EOS 600D with 60 mm f/2.8 EF-S macro lens)
Slides of ichneumonids by Ernie Ives (Canon EOS 600D with 60 mm f/2.8 EF-S macro lens)
Discussion at the gossip provided an answer: the slides appear to be Ernie’s early experiments with the Ralmount mountant.
 Ichneumonid on a slide by Ernie Ives (body length 2.1 mm, 2× objective, 2.5× photo eyepiece, Canon EOS 5D Mark II, EOS Utility)
Ichneumonid on a slide by Ernie Ives (body length 2.1 mm, 2× objective, 2.5× photo eyepiece, Canon EOS 5D Mark II, EOS Utility)
 Spacers supporting the coverslip, seen through the slide (Canon EOS 600D with 60 mm f/2.8 EF-S macro lens)
Spacers supporting the coverslip, seen through the slide (Canon EOS 600D with 60 mm f/2.8 EF-S macro lens)
July 2020
I have finally had time to get the Olympus infinity-corrected metallurgical objectives and brightfield vertical illuminator that I acquired in October 2016 working with the TH transformer (modified to take 4 mm banana plugs) that I acquired in October 2018. The lamphouse uses a cable that only fits the TGH transformer, and I have failed to find one at a reasonable price. Despite having exploded parts diagrams for the lamphouse and the plug, I could not get access to the wires at either end of the cable, so I reluctantly cut it and then fitted an extension with heavy-duty Deltron bunch pin plugs.
 Olympus BHT with BH2-MA vertical illuminator and MSPlan objectives
Olympus BHT with BH2-MA vertical illuminator and MSPlan objectives
 Olympus TH transformers (left one modified to accept banana plugs)
Olympus TH transformers (left one modified to accept banana plugs)
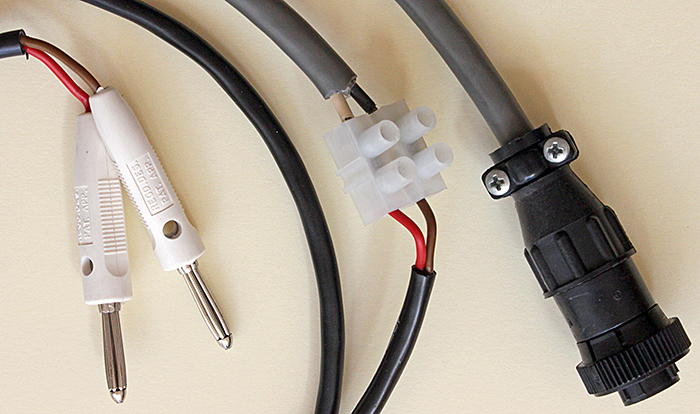 Cable from BH2-MLSH lamphouse with bunch pin plugs (original plug on right)
Cable from BH2-MLSH lamphouse with bunch pin plugs (original plug on right)
One of the first things I tried was a human grey scalp hair, because I was trying to get a photo of the scales on the cuticle for the July “Hairs and fibres” gossip (our first Zoom meeting). The 20× MSPlan gave better results than my 20× Neo (from the BH era) but not as good as my 20× SPlan with transmitted light. I took some photographs with normal light and with crossed polarisers (the cortex of human hair is birefringent) and showed them during the gossip.
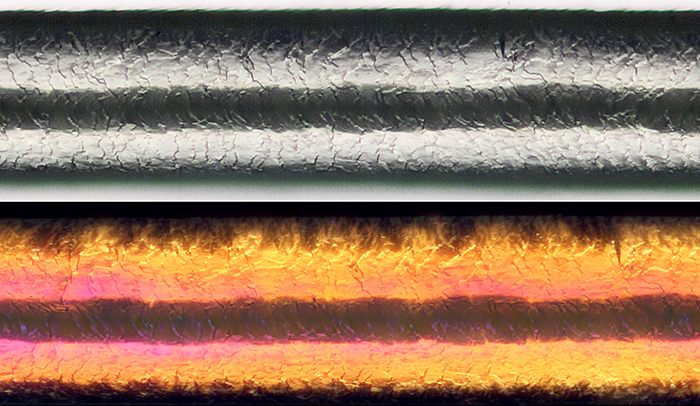 Scales on the cuticle of human grey scalp hair (hair diameter 0.092 mm, with crossed polarisers in the lower photo, 20× objective, 2.5× photo eyepiece, Canon EOS 5D Mark II, EOS Utility)
Scales on the cuticle of human grey scalp hair (hair diameter 0.092 mm, with crossed polarisers in the lower photo, 20× objective, 2.5× photo eyepiece, Canon EOS 5D Mark II, EOS Utility)
I don’t make permanent slides, so to hold the hairs straight I stretched them across an aluminium cell slide and fastened them with Mineral Tack.
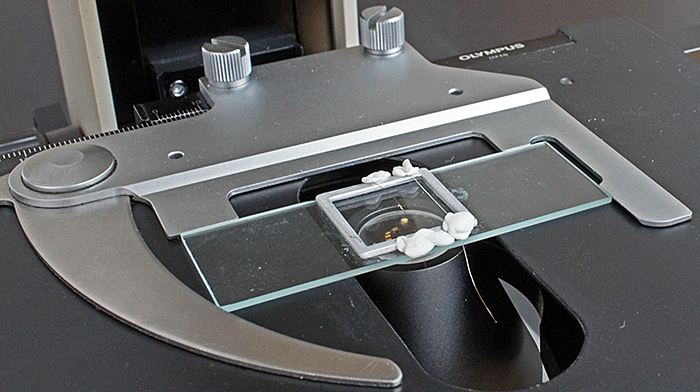 Holding hairs straight
Holding hairs straight
Using polarisers gave me an idea for a presentation on getting started with polarised light microscopy for the next Zoom gossip, the joint meeting with the Bournemouth Natural Science Society. I showed how to use a photographic polarising filter as the polariser, a disk of polarising film as the analyser, and all sorts of plastic film as a retarder. I have been accumulating pieces of plastic film to test as retarders, and I devised an annular mount to make it easy to hold and rotate them; it was easy to make with a compass cutter.
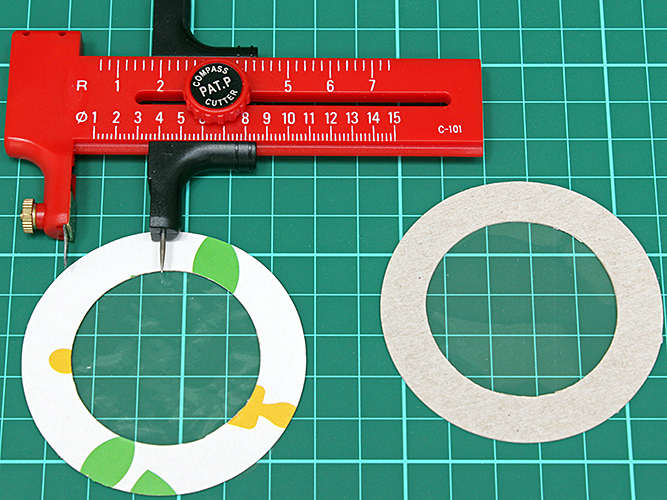 Making annular mounts with a compass cutter
Making annular mounts with a compass cutter
In addition to the PowerPoint, I showed a live view through my microscope of adjusting the polariser for extinction, rotating the slide for the best image, and using a retarder to change the background and specimen colours.
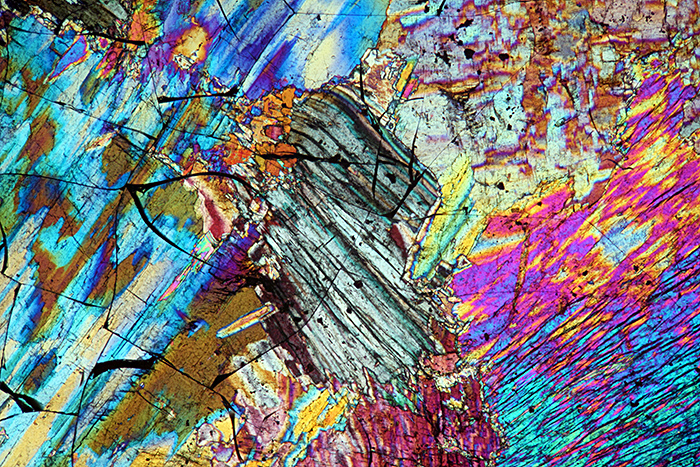 Thin section of sillimanite (crossed polarisers plus retarder, 4× objective, 2.5× photo eyepiece, Canon EOS 5D Mark II, EOS Utility)
Thin section of sillimanite (crossed polarisers plus retarder, 4× objective, 2.5× photo eyepiece, Canon EOS 5D Mark II, EOS Utility)
June 2020
With no meetings to go to, I have finally had time to clear the backlog of Olympus microscope instruction manuals, brochures, catalogues, exploded parts diagrams and repair manuals that I wanted to scan and convert to PDFs so that they can go on my website (www.alanwood.net/olympus/downloads.html). Some of them came from Brian Bracegirdle’s estate, some were donated by other people, and others came from Club meetings.
 Scanning catalogues
Scanning catalogues
 Scanned Olympus documents
Scanned Olympus documents
I have also had time to work out how to update the Quekett website to use HTTPS (Hypertext Transfer Protocol Secure) so that any passwords that members enter and any messages that people send are transmitted securely. You can check that the website is secure by looking for a closed padlock icon in the address bar of your web browser:
 Closed padlock icon in Google Chrome browser
Closed padlock icon in Google Chrome browser
Pages on the Quekett website should also load faster, because web browsers use the faster HTTP/2 network protocol with websites that use HTTPS. It is not easy to prove that HTTP/2 is being used, but I found a Firefox extension (HTTP/2 Indicator) that puts a blue lightning bolt in the address bar:
![]() Blue lightning bolt in Firefox browser
Blue lightning bolt in Firefox browser
May 2020
On Saturday 2nd May I received an e-mail from the Rangers Office on Wimbledon Common asking me to phone them, and I was devastated to hear that Dennis Fullwood had died. The neighbour who sounded the alarm did not know how to contact his family, but she knew he was involved with the Rangers. They had my e-mail address because I send them photographs of Quekett activities on the common. Nobody in the Quekett or the Natural History Museum or the Kent Geologists’ Group knew of any family except an aunt in a care home somewhere. Lisa Ashby did a brilliant piece of detective work on ancestry.co.uk and traced members of the Fullwood family in Canada who knew how to contact a relative in Carshalton, so now we will be informed about funeral arrangements and Dennis’s estate can go to family members.
Kai and I miss being able to catch a bus and go for long walks on Box Hill, Headley Heath and Mickleham Downs, but the bus ride does not qualify as a necessary journey. For exercise we are combining shopping with walks to the neighbouring towns, a round trip of about 5 miles.
Finally finished Amendment 7 to ISO 1750, after spending several days fixing all of the problems that ISO’s Word template introduced. It includes well over 100 entries like this, all meticulously checked and re-checked:
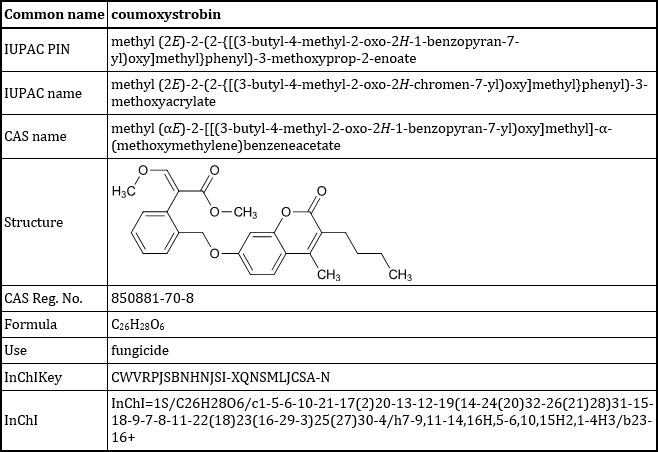 Coumoxystrobin table for ISO 1750
Coumoxystrobin table for ISO 1750
Now I hope to have some time for microscopy. There is no news of when we will be able to have normal microscope club meetings, but we have held a couple of committee meetings using Zoom, and we are hoping to use Zoom for gossip meetings and lectures. We are also working on contingency plans for a virtual annual exhibition.
April 2020
No microscope club meetings to go to, and no meeting reports to write, so I have finally have the time to work on an amendment to add more pesticide common names to ISO 1750 Pesticides and other agrochemicals – Common names. I really want to give this up so that I have more time for microscopy, but it is hard work trying to get ISO to set up a database of pesticide names.
March 2020
I missed the AGM and the Reading Convention because we were in Thailand for our annual visit to pay our respects at the graves of Kai’s parents in a huge Chinese cemetery that used to be a rice field. The lockdown in the UK and the cancellation of most flights in and out of Thailand started while we were there, but fortunately our Thai Airways flight was not cancelled, although it was completely full because BA had abandoned their passengers. We came back to a very different world, not much traffic on the roads, queues to get into supermarkets, and no microscope club meetings.
February 2020
Doug Richardson bequeathed his collection of nearly 2500 slides to the Quekett so that some of them could be added to the loan sets that members can borrow. There are lots of slides of insects and their parts, and many other invertebrates, birds, fishes, reptiles, mammals, plants, fibres and crystals. Nearly all of the slides were made by Doug himself, but there are a few by other people, including Brian Darnton, Vaughan Dodge, Dave Skeet and E. Markham.
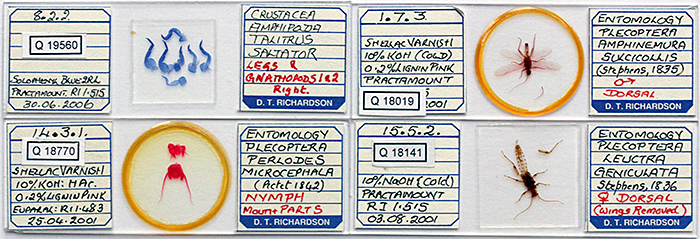 Slides by Doug Richardson
Slides by Doug Richardson
 Dry mounts and deep mount by Doug Richardson
Dry mounts and deep mount by Doug Richardson
Almost all of the slides have been photographed and have had Quekett slide numbers added, and a workshop was held on how volunteers can help transcribed the information on the labels into an Excel spreadsheet. The photos and data will then be added to the database of the Club’s slide collection. The participants were allowed to select any slides they liked to take home and transcribe the data.
January 2020
I haven’t acquired anything new for some time, so for the “My latest microscopical acquisition” I showed the 2 ways I have been testing to photograph slides through microscopes at Club meetings. The more promising seems to be afocal coupling, with a Leitz Periplan 10×/18 eyepiece (from Microscopium) connected via a step-down filter adapter (from eBay) to an Olympus Zuiko 50 mm standard lens from the OM system, with an OM-EOS adapter (from eBay). This arrangement is rigid and parfocal. The other way is eyepiece projection, with a simple microscope adapter (from eBay) from the days of 35 mm SLR cameras that clamps to an eyepiece tube and holds a normal eyepiece, connected to a camera with a T-mount.
 Afocal coupling (left) and eyepiece projection adapters
Afocal coupling (left) and eyepiece projection adapters
About this blog
I am Alan Wood, former webmaster for the Quekett website, and author of several web pages on Olympus microscopes. I spend too much time writing about microscopes and buying more equipment. I hope this blog will help me to focus on using my microscopes so that I have something to write about!
If you would like to comment on anything in this blog, please contact Alan Wood.

