Starting digital photomicrography
Introduction
Photomicrography – the process of taking photographs with the microscope – used to be a highly-specialised skill taking many years of practice to achieve good results. Digital cameras have largely changed that, as the instant viewing of results allows errors to be corrected immediately.
This introduction aims to help those with limited skills or confidence to get started in photomicrography, which can now be performed at relatively low cost and with minimal equipment.
Good microscope technique is the essential starting point to taking good photomicrographs. With the compound microscope, the aims in setting up the microscope are to achieve even illumination and an acceptable level of contrast with the subject; the human eye can correct for a lot of defects automatically which the digital camera faithfully records!.
Achieving even illumination
Modern microscopes with built-in illumination make achieving even illumination much easier than the old mirror and separate lamp arrangements. The procedure for setting up Köhler illumination, the standard method for achieving even illumination, is described in most microscope manuals. Simply focus on the specimen with both the substage condenser iris and the field lens iris (the one closest to the lamp) open. Partially close the field iris and focus the condenser down from its top position until the edges of the field iris are sharply focused in the same field as the specimen. Now open the field iris until its perimeter is just outside the field of view. Now remove one eyepiece and, looking down the eyetube, partially close the condenser iris until it blocks approximately one quarter to one third of the field of view. This is the optimum setting for contrast in brightfield conditions. Note that some simpler (but still perfectly capable) microscopes do not have a field iris; in this situation a ground glass filter is usually present close to the bulb to diffuse the illumination.
Achieving contrast
This subject alone fills several books and cannot be covered to any extent in this introduction. However, achieving good contrast with many subjects is important to produce good results. Brightfield conditions can work well if the subject is of high contrast. However, many organisms are very transparent and subsequently of low contrast. For these subjects, darkground and Rheinberg illumination work well and produce attractive results.
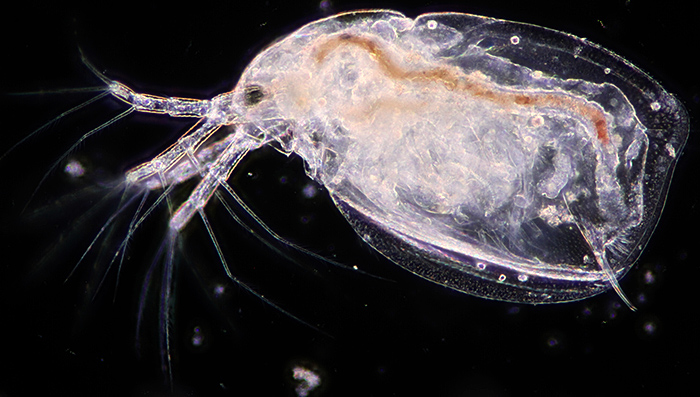 Figure 1: Darkground illumination
Figure 1: Darkground illumination
Darkground (figure 1) is achieved either by use of a special darkground condenser (usually a cardioid mirror) or (for objectives up to ×40) with a patch stop in the condenser filter tray. If you have a phase contrast condenser, the phase ring for a higher magnification objective often works well as a darkground stop.
 Figure 2: Rheinberg illumination
Figure 2: Rheinberg illumination
Rheinberg illumination (figure 2) can produce beautifully coloured images, with the background one colour and the waterflea in a contrasting colour. It is achieved using a filter with an inner circle of one colour (forming the background) and an outer ring of the complementary colour. Phase contrast is a widely-available system for increasing contrast but does require some adjustment to get the best results.
Finally, for the well-funded, Differential Interference Contrast (also known as DIC or Nomarski after its inventor) provides superb contrast – but at a price!
Choice of camera
Often the choice is already determined by what is already available! The easiest route is certainly a camera with a removable lens – typically a DSLR camera. More recent models have tilting viewing screens which can help with framing and focusing (rather than climbing a ladder to see down a camera mounted on top of a tall microscope!) but the best solution if the camera is capable, is to use ‘tethered’ shooting, driving the camera and viewing the image directly from a computer via a USB lead. Canon cameras in particular are very easy to use in this respect.
Cameras with fixed lenses can be used but require more thought in how to couple the camera to the microscope.
 Figure 3: Eyepiece attached to a compact camera
Figure 3: Eyepiece attached to a compact camera
Some have a filter screw thread at the front of the lens which can be coupled to an eyepiece with the same screw thread (figure 3) or the lens can simply be held in place over the eyepiece with a home-made support bracket.
Coupling camera to microscope
Both the microscope and camera need suitable fittings for the two to be coupled together.
With compound microscopes, the easiest option is to use a trinocular tube (providing binocular tubes for viewing and a third vertical tube for permanent attachment of the camera). Trinocular tubes contain a prism that diverts either all or some of the light to the camera; more advanced fittings have a slider that allows different division of the light to either the eyes or camera. A much lower cost solution, fine for occasional photography, is a simple vertical monocular tube that replaces the normal angled viewing head. Finally, it is possible to couple a camera directly onto one of the angled eyepiece tubes of a binocular head as a temporary set-up; heavier cameras make this more difficult and a stable (heavy) microscope is required.
With stereomicroscopes, the situation can be more difficult. Stereos can be supplied or fitted with trinocular heads but these are expensive and hard to find second-hand, whilst the eyepiece tubes are generally of wider diameter (e.g. 30 mm) and do not fit the widely-available microscope to camera adapters.
The camera will need a microscope adapter to connect to the microscope tube.
 Figure 4: Digital SLR with a camera adapter
Figure 4: Digital SLR with a camera adapter
These are readily available second-hand (either from the adverts in Amateur Photographer or via eBay); it is best to find one with a T-2 thread mount (such as the Pentax adapter; figure 4) which can then be mounted onto the camera bayonet mount with a readily available T-2 adapter (again via eBay). New versions can also be bought from SRB-Griturn.
The final consideration is the eyepiece to be used between the microscope and camera. A normal viewing eyepiece can be used but will require the microscope to be significantly defocused to achieve focus at the camera. This can often be reduced or eliminated by slightly raising the eyepiece in its tube by about 5 mm with a card or paper collar; some experimentation will be needed to find the most amenable eyepiece and its best position. If available, chose a lower power eyepiece to achieve a wider field of view on the camera. Specialist photographic eyepieces, usually of low power, are also available; the Olympus versions work well in most circumstances and are often available second-hand.
Taking the photograph
Stationary subjects are much easier to cope with! Water fleas can be killed easily or narcotised with Boots brand Anaesthetic Throat Spray (containing lidocaine).
The camera, with lens removed, should be set to Shutter Speed control (or fully manual) and typically set around 1/15 of a second to start with. Film speed (if selectable) should be set to a low ASA e.g. 100 or 200 and white balance to automatic. The big issue in taking a photomicrograph is preventing vibration from the camera mirror, which is magnified by the microscope and results in ‘blurry’ images. Many DSLRs have a ‘mirror lock’ option in their menus, or the mirror will already be up if shooting from ‘Live View’ mode (which makes framing and focus much easier). Even now, the process of touching the shutter button will cause vibration and must be avoided. The options are either to use an electronic shutter release cable, or to use camera software to ‘fire’ the camera from a computer.
Reviewing the results & correcting defects
The great magic of digital photography is that results can be viewed immediately and defects corrected. Common problems:
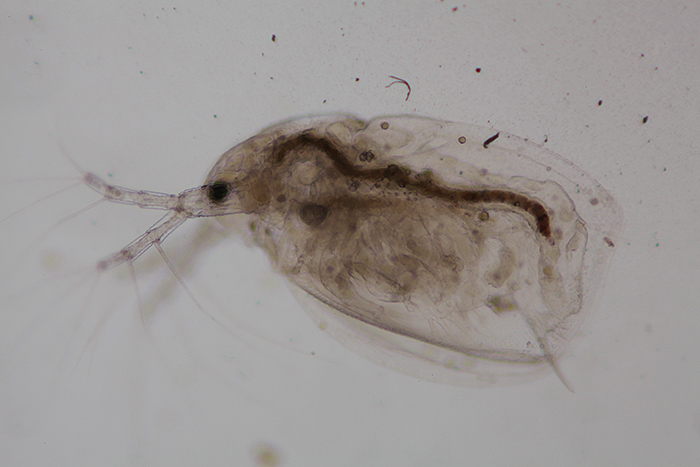 Figure 5: Dirt in the field of view
Figure 5: Dirt in the field of view
Dirt in the field of view (figure 5) – the usual locations for dirt are the top lens of the condenser and the top of the eyepiece. Condenser dirt can be detected (and eliminated from the photo) by slightly defocusing (lowering) the condenser. In both instances clean with a blower brush and lens cleaning solution.
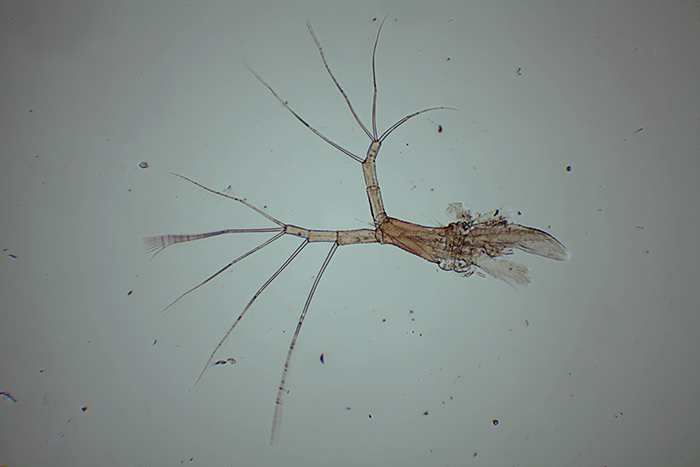 Figure 6: Uneven illumination
Figure 6: Uneven illumination
Uneven illumination (figure 6) – Problems here can be difficult to detect as there are several possible causes. Check firstly that the microscope is set up correctly and that the condenser filter tray has not been pushed partially across the illumination path. Other problems may be caused by a non-aligned component of the microscope (such as the condenser). The eyepiece used to project the image to the camera may also be a cause of ‘flare’ – trial and error with different eyepieces may be needed.
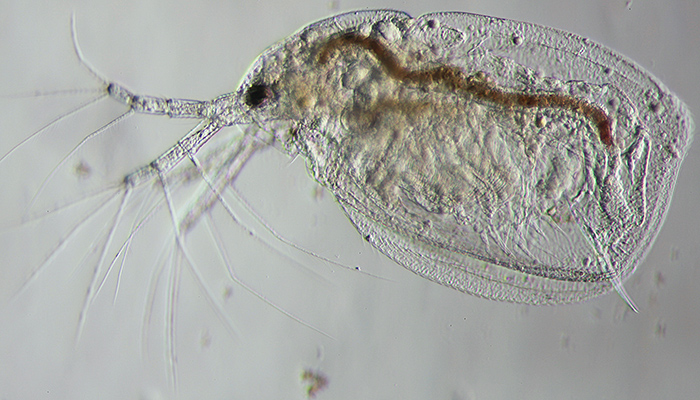 Figure 7: Grainy appearance
Figure 7: Grainy appearance
‘Grainy’ appearance (figure 7) – Results from having the condenser iris closed down excessively. Open it to no more than one third of the aperture of the objective when looking down an eyetube with the eyepiece removed.
Like most skills, perfection comes with practice, and at least with digital cameras trial and error costs nothing.
Want to learn more?
The Resources section of our website contains articles on particular aspects of digital photomicrography, including scale bars, stacking and stitching, as well as an article on advanced digital photomicrography.
David Linstead, one of the Club’s most experienced photomicrographers, has produced the following introduction to photography through the microscope. It is formatted as a slide show, so use the arrow keys on your keyboard to go from one slide to the next.

