Bdelloid rotifers
An introduction to bdelloid rotifers and their study
Aydin Örstan
Germantown, Maryland, U.S.A.
Introduction
Bdelloid rotifers are microscopic aquatic animals that are easily recognized by the pair of ciliated, eversible disks (corona) that most species have on their heads and their characteristic creeping like an inchworm or a leech. Besides their interesting morphologies, bdelloids also boast unusual biological traits, including exclusively parthenogenetic reproduction and the ability to survive drying, that altogether make them unique among all animals. Even so, bdelloids seem to be some of the least favorite subjects of microscopists.
Admittedly, bdelloids are difficult to study. The identification of almost all species requires that the animal be alive and active with its corona unfolded. But active bdelloids are often unruly and hence difficult to follow under the microscope, especially at high magnifications necessary to examine taxonomically significant body parts. At the same time, even the slightest disturbance may cause them to contract their bodies into unidentifiable balls and to remain so for frustratingly long periods. To make matters worse, there are no readily available reagents that can be used to anesthetize them and, until recently, there was no satisfactory method to preserve their bodies extended.
Needless to say, these challenges have been readily accepted and overcome by many microscopists over the years. Two pioneering rotiferologists of the early 20th century, James Murray (1865–1914) and David Bryce (1853–1934) were both amateur members of the Quekett Microscopical Club. The numbers of bdelloid species Murray and Bryce described add up to about 30% of the current total of about 400.
I hope that this brief guide will increase interest in these animals that certainly deserve more attention from microscopists.
Phylogeny of bdelloids
Bdelloids (Bdelloidea) along with monogononts (Monogononta) and seisonids (Pararotatoria) are in the phylum Rotifera. On a higher level of evolutionary classification, rotifers are in a group of mostly microscopic, aquatic animals known as the Gnathifera (Hejnol, 2015)[9]. The other members of the Gnathifera are the gnathostomulids, micrognathozoans and acanthocephalans. The phylum Gnathostomulida contains interstitial, worm-like creatures that live in the sea. The taxon Micrognathozoa consists of but one species that lives in freshwater streams on the islands near Greenland. The Acanthocephala, some species of which can be several tens of centimeters long, are internal parasites of vertebrates. They appear to be related more closely to the rotifers than to the other gnathiferans (Fontaneto & De Smet, 2015)[7]. All gnathiferans, except the acanthocephalans, possess a hard jaw-like organ. Among the gnathiferans, only the bdelloid rotifers have species that can survive drying as adults.
Anatomy of bdelloids
Most species are about 200 to 500 µm long. But a few, for example, Rotaria neptunia, can be longer than a millimetre (Fig. 1). The body of a bdelloid has been divided traditionally into five parts (Fig. 2): head, neck, trunk, rump and foot (Donner, 1965[5]; Iakovenko et al., 2015[10]). Although there is no internal segmentation, the integument, especially around the foot, has well-defined transverse pseudosegments (Fig. 2). These pseudosegments render the body fully telescopic; a bdelloid can contract tightly into a shape that resembles, especially when dry, a microscopic lemon (Fig. 3). The integument of most species is transparent, but in some species the trunk accumulates a coat of debris that obscures the internal organs. Several species have on their bodies variously shaped spines, appendages or protuberances of unknown functions (Fig. 4).
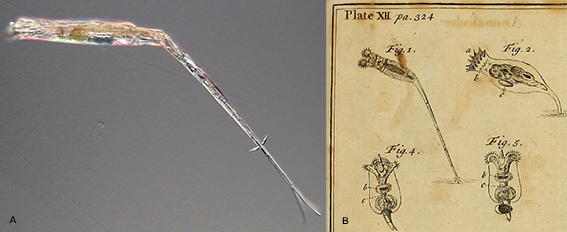 Figure 1. A: Rotaria neptunia has an unusually long and narrow foot. The total length of this individual exceeded 1 mm. B: Detail from a plate in Baker (1764)[1] showing some of the rotifers he had observed. He described the rotifers in Fig. 1 as having “Tails enormously long”. They were probably Rotaria neptunia, the first time this species was noted and drawn.
Figure 1. A: Rotaria neptunia has an unusually long and narrow foot. The total length of this individual exceeded 1 mm. B: Detail from a plate in Baker (1764)[1] showing some of the rotifers he had observed. He described the rotifers in Fig. 1 as having “Tails enormously long”. They were probably Rotaria neptunia, the first time this species was noted and drawn.
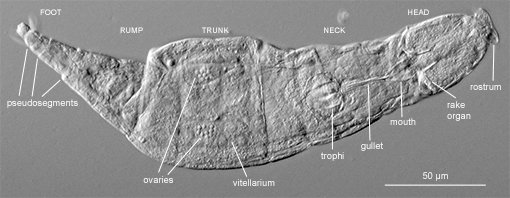 Figure 2. Various body parts are visible in this photograph of an Adineta (DIC).
Figure 2. Various body parts are visible in this photograph of an Adineta (DIC).
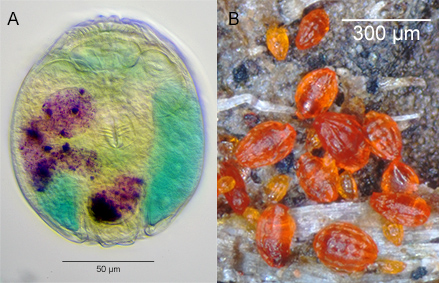 Figure 3. A: A preserved contracted Philodina specimen. Before it was killed and fixed with hot glutaraldehyde, the rotifer had been fed carmine, the particles of which now mark the twisting lumen of its stomach. The trophi are almost at the center and the two ciliated disks of the corona are above it. The vitellaria, on one each side, were dyed with methylene blue (oblique illumination). B: Dry bdelloids of different species attached to a bit of debris removed from a dry birdbath.
Figure 3. A: A preserved contracted Philodina specimen. Before it was killed and fixed with hot glutaraldehyde, the rotifer had been fed carmine, the particles of which now mark the twisting lumen of its stomach. The trophi are almost at the center and the two ciliated disks of the corona are above it. The vitellaria, on one each side, were dyed with methylene blue (oblique illumination). B: Dry bdelloids of different species attached to a bit of debris removed from a dry birdbath.
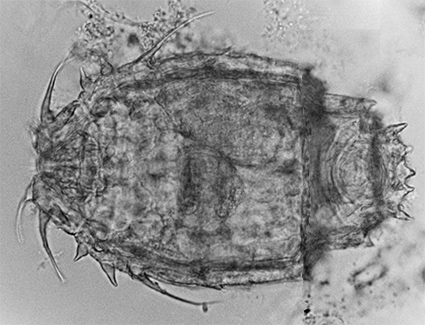 Figure 4. A contracted Macrotrachela multispinosa with multiple spines on its trunk (brightfield; composite of 2 images).
Figure 4. A contracted Macrotrachela multispinosa with multiple spines on its trunk (brightfield; composite of 2 images).
The majority of the bdelloid species are in the families Habrotrochidae and Philodinidae and carry on their heads the characteristic corona consisting of two ciliated disks on pedicels (Fig. 5A). The mouth is a funnel-like ciliated structure posterior to the corona on the ventral side of the head. The corona and the mouth open only during swimming or stationary feeding. During creeping, the corona is withdrawn into the head, the mouth is closed, and a proboscis-like rostrum with a ciliated tip becomes the front end (Fig. 5B). The taxonomically significant upper lip is the dorsal area visible between the pedicels of the open corona and the partially withdrawn rostrum (Figs. 5A, 6). In the family Adinetidae, the head is flattened and the corona is in the form of a flat ciliated surface covering most of the ventral side of the head (Fig. 7). On the dorsal surfaces of the heads of all species there is an antenna that varies in length.
 Figure. 5. A: A Philodina feeding (DIC). Its upper lip (arrow) consists of several layers of integument. The enlarged vitellarium occupies most of the right half of the trunk and a developing egg the left half. B: Same animal crawling with its corona withdrawn and rostrum extended.
Figure. 5. A: A Philodina feeding (DIC). Its upper lip (arrow) consists of several layers of integument. The enlarged vitellarium occupies most of the right half of the trunk and a developing egg the left half. B: Same animal crawling with its corona withdrawn and rostrum extended.
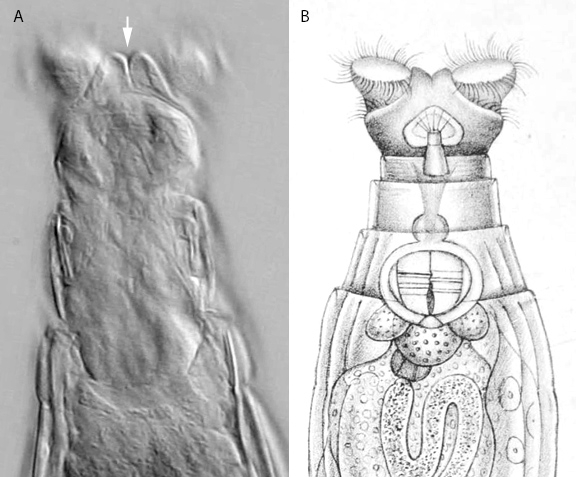 Figure 6. A: A frame extracted from a high-resolution video of a feeding Macrotrachela cf. habita. Its double-lobed upper lip (arrow) is distinct (oblique illumination; contrast-enhanced). B: The drawing of the species in David Bryce’s description from 1894[2].
Figure 6. A: A frame extracted from a high-resolution video of a feeding Macrotrachela cf. habita. Its double-lobed upper lip (arrow) is distinct (oblique illumination; contrast-enhanced). B: The drawing of the species in David Bryce’s description from 1894[2].
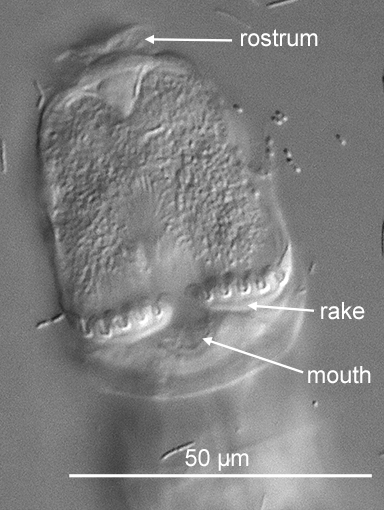 Figure 7. The ventral side of the head of an Adineta. The number of the tiny shovel-like denticles on each rake (in this case six) is always one more than the number of the U-shaped gaps (five) in between the denticles (DIC).
Figure 7. The ventral side of the head of an Adineta. The number of the tiny shovel-like denticles on each rake (in this case six) is always one more than the number of the U-shaped gaps (five) in between the denticles (DIC).
The jaws of bdelloids, known as the trophi, are easily noticed thanks to their almost incessant movement when a rotifer is feeding. The early microscopist Baker (1764)[1] mistook the trophi for a heart and was confused when he observed that the “heart” sometimes suspended its motions as the trophi do when a bdelloid is not feeding (in reality, because of their small size, there is no circulatory system in rotifers). The trophi consist of several hard parts surrounded by muscles that are collectively known as the mastax. The upper surface of the trophi, about 10 to 35 µm long, is lined with numerous ridges functioning as teeth (Fig. 8). There are major and minor teeth, distinguished by their widths, and sometimes ancillary teeth of an intermediate width. The number of major teeth on each half, traditionally expressed formulaically as, for example, 2/2, when there are two major teeth on each side, varies from one to ten depending on the species (Melone & Fontaneto, 2005)[13]. The dental formula is a useful taxonomic character. In addition, the presence of interproximal teeth between the major teeth in some species was noted recently (Örstan, 2020a[18]). Adinetid species also have, in addition to their trophi, a rake organ consisting of tiny shovel-like denticles lined transversely on movable plates on each side of the entrance to their mouths (Fig. 7).
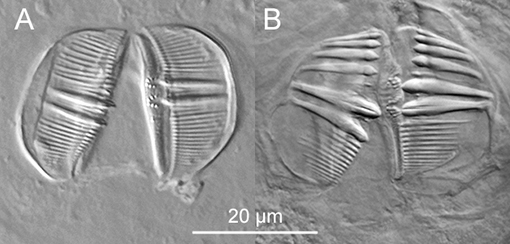 Figure 8. Trophi of bdelloids (DIC). A: Trophi of a Pseudoembata; dental formula: 2/2. B: Trophi of an Abrochtha; dental formula: 6/6.
Figure 8. Trophi of bdelloids (DIC). A: Trophi of a Pseudoembata; dental formula: 2/2. B: Trophi of an Abrochtha; dental formula: 6/6.
Bdelloids, being bilaterally symmetric, have either paired organs placed more or less laterally or single medially placed organs. Located in the head dorsoanterior to the trophi is the relatively large brain (Fig. 9). Several additional bag-like organs in the head and the neck have traditionally been considered to be glands, although their exact functions are unknown. The most prominent of them, often almost as large as the brain, is the dorsotransverse gland placed dorsal to the beginning of the stomach (Fig. 9). The pair of eye spots, when present, are always under the integument either in the brain or in the rostrum (Figs. 10, 14). The internalization of the eye spots, as opposed to being on the external surface, makes it unlikely that they can form images. They probably function simply to detect light. Bdelloid species with and without eye spots often share the same habitat. The possible differences between the spatial distributions of such species within their habitats and their behaviors are not known.
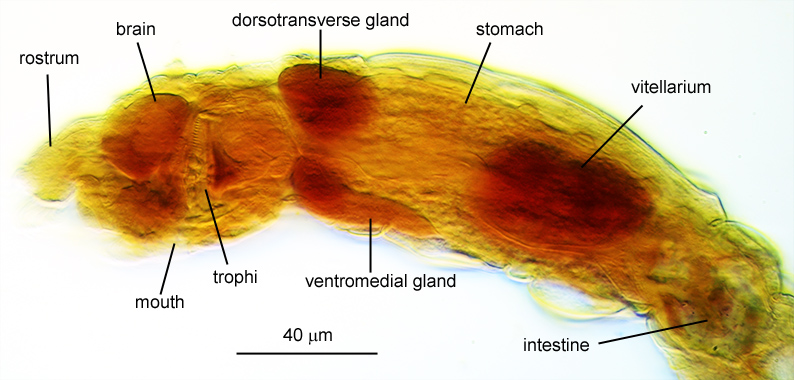 Figure 9. The lateral view of a preserved specimen of an Abrochtha showing the major organs of the head and the trunk. The rotifer was killed with hot glutaraldehyde, stained with neutral red and mounted in glycerine jelly (image was processed to accentuate the colors; oblique illumination).
Figure 9. The lateral view of a preserved specimen of an Abrochtha showing the major organs of the head and the trunk. The rotifer was killed with hot glutaraldehyde, stained with neutral red and mounted in glycerine jelly (image was processed to accentuate the colors; oblique illumination).
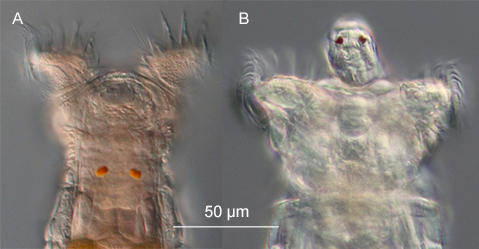 Figure 10: Eye spots of a Philodina (A) and Rotaria tardigrada (B) (DIC).
Figure 10: Eye spots of a Philodina (A) and Rotaria tardigrada (B) (DIC).
In the families Adinetidae, Philodinidae, Philodinavidae and the recently described Coronistomidae a ciliated lumen goes through the stomach, but is absent in the Habrotrochidae. The stomach connects to an intestine, which is followed by a contractile cloaca in the rump. The protonephridial system, responsible for excretion and osmotic regulation, consists of a tubule along each side of the body extending from the head into the cloaca. There are several elongated cells attached to the tubules. These are called flame cells because the movement of the bundles of cilia inside them creates the impression of a flickering flame. The movements of these cilia, visible at high magnifications, are sometimes the only indication of life in otherwise contracted and motionless bdelloids.
The trunk also contains a pair of vitellaria (yolk glands) each with an associated ovary. One vitellarium is often larger than the other and is easily recognized by the large nuclei it contains, which are eight in number in most species (Fig. 5A). Ovaries consist of a number of small cells and are usually difficult to see. Perhaps because they have flatter bodies, the ovaries of Adineta are easier to spot (Fig. 2). Some organs of bdelloids, including the integument and the vitellaria, are syncytial in which the nuclei are distributed without cell membranes.
In most species there is a pair of appendages called spurs near the end of the foot. The spurs seem to contribute to the attachment and the balancing of the animal during feeding. The foot ends with two to four toes or an adhesive plate (Fig. 11).
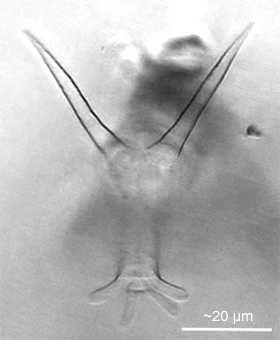 Figure 11: A frame extracted from a high-resolution video of a Dissotrocha macrostyla showing the long spurs that give the species its specific name and the four toes at the end of the foot. The rotifer was creeping on the underside of a cover glass (oblique illumination; contrast-enhanced).
Figure 11: A frame extracted from a high-resolution video of a Dissotrocha macrostyla showing the long spurs that give the species its specific name and the four toes at the end of the foot. The rotifer was creeping on the underside of a cover glass (oblique illumination; contrast-enhanced).
Reproduction
All bdelloids taken from the wild or from laboratory cultures are expected to be females. There have been no definitive observations of male bdelloids and they are, therefore, assumed not to exist. The only pertinent record was that of Wesenberg-Lund (1930)[24] who claimed to have seen on two occasions one male among thousands of Rotaria rotatoria, but failed to isolate and study them. It is possible that male bdelloids do exist, but are rare, seasonal and short-lived.
In laboratory cultures female bdelloids produce unfertilized eggs that develop into females. This process is called parthenogenesis. Bdelloids are the largest group of eukaryotes that appears to reproduce exclusively by parthenogenesis. However, the question of how bdelloids may have survived over evolutionarily significant periods without exchanging genes still remains unanswered. In a recent unpublished study, Laine et al. (2020)[11] argued for the presence of sexual reproduction in bdelloids based on genetic evidence.
Most bdelloid species are oviparous. Eggs are about 50-100 µm long. The eggs of most species have shells that appear smooth at the magnifications of light microscopes, but some eggs are covered with protuberances of various shapes (Fig. 12). There are also viviparous (strictly speaking, ovoviviparous) species that give birth to fully developed young. For example, all of the species in the genera Rotaria and Dissotrocha are viviparous. Rotifers carrying advanced embryos are easily recognized by the second trophi that will be visible in their trunks (Fig. 13).
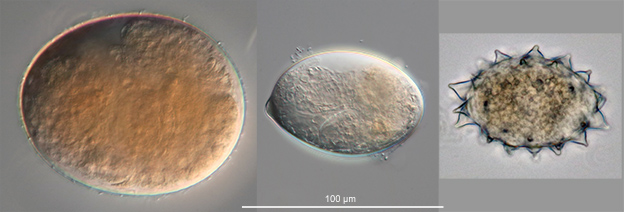 Figure 12. Eggs of bdelloid rotifers. All to the same scale.
Figure 12. Eggs of bdelloid rotifers. All to the same scale.
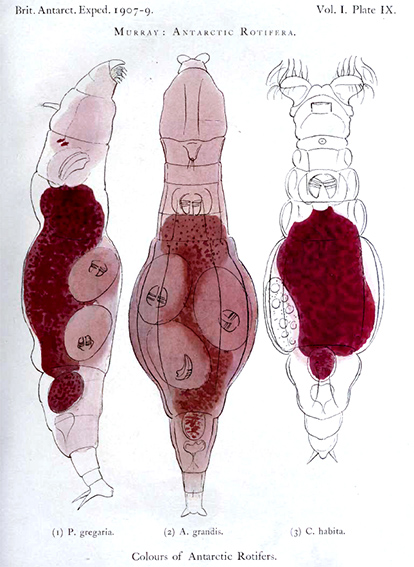 Figure 13. Color plate from James Murray’s 1910 report[14] showing some of the bdelloid rotifers of Antarctica he studied during the British Antarctic Expedition of 1907–9. Murray thought the red color of the animals’ stomachs was probably related to their food. Both Philodina gregaria and Adineta grandis are viviparous as indicated by the trophi of the multiple embryos they are carrying.
Figure 13. Color plate from James Murray’s 1910 report[14] showing some of the bdelloid rotifers of Antarctica he studied during the British Antarctic Expedition of 1907–9. Murray thought the red color of the animals’ stomachs was probably related to their food. Both Philodina gregaria and Adineta grandis are viviparous as indicated by the trophi of the multiple embryos they are carrying.
Habitats and drying survival
Bdelloids are common and widespread in freshwater habitats from where the majority of the known species have been described. Bdelloids may be found not only in rivers, lakes and ponds, but also in habitats that one may not think of associating with freshwaters. The latter include mosses and lichens growing on rocks, trees and rooftops; rain puddles, rock pools, bird baths and even the tanks of sewage treatment plants. In fact, bdelloids were among the first “animalcules” the pioneering microscopist Leeuwenhoek discovered in the rain gutters of his house (Ford, 1982)[8]. Although one may not expect a place like Antarctica to have a rich diversity of invertebrates, there are in fact several species of bdelloids that are endemic to that continent (Iakovenko et al., 2015)[10]. For example, one of the most common invertebrates in the meltwater ponds in Antarctica is the bdelloid Philodina gregaria that was discovered by James Murray during the British Antarctic Expedition of 1907–9 (Fig. 13). Murray (1910)[14] could obtain large numbers of these rotifers in the winter by thawing ice containing plants from the lakes.
Several species in the genus Habrotrocha spend their lives inside the cases they build for themselves and some species take refuge in nests of debris (Fig. 14). Most bdelloids are free living, but some are epizoic on freshwater arthropods. Likewise, the only one truly marine bdelloid, Zelinkiella synaptae, is epizoic on sea cucumbers. This puzzling jump from a widespread presence in freshwaters to the epizoic lifestyle of a single species in the sea leaves one all the more curious about the group’s evolutionary history.
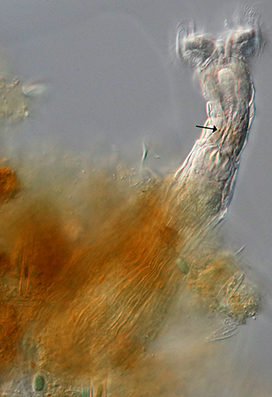 Figure 14: Habrotrocha cf. collaris feeding out of its nest of debris (DIC). Its faint orange eye spots (arrow) are barely visible.
Figure 14: Habrotrocha cf. collaris feeding out of its nest of debris (DIC). Its faint orange eye spots (arrow) are barely visible.
The members of the genera Henoceros and Philodinavus, which are found only in running waters, display rather unique morphologies not present in species that live in other types of habitats. This suggests a possible evolutionary correlation between their habitats and morphologies. Recently, a new bdelloid, Coronistomus impossibilis, was described from a river in Maryland, U.S.A. (Örstan, 2021)[21]. The morphology of the new species was so unusual that it was necessary to erect not just a new genus but also a new family to accommodate it. Microscopists are urged to search fluvial habitats for species with novel morphologies that may lead to a better understanding of the origin and the evolution of the bdelloids.
If dry pieces of mosses, lichens, dry mud from recurring rain puddles or dry debris from birdbaths and similar water receptacles are placed in clean water, live bdelloids are usually obtained (Örstan, 1998)[15]. Rehydration of dry mosses is indeed a time-honored method of obtaining bdelloids and many new species have been described from such samples. This is a simple demonstration that the bdelloids that live in such ephemeral habitats can and necessarily survive drying. The bdelloids that inhabit birdbaths appear to be especially tolerant of drying. If a birdbath is allowed to dry out during a rainless period, enormous numbers of brightly colored dry bdelloids may accumulate on bits of debris (Figs. 3B, 15). Upon placement in water, the majority of these rotifers revive. The birdbath bdelloids also tolerate exposure to solar radiation, water temperatures at least as high as 42°C as well as freezing (Örstan, 2020c)[20]. I have been conducting a long-term experiment to monitor the survival of dry birdbath bdelloids in the freezer of a kitchen refrigerator at −21°C. In a sample rehydrated after five years, 98% of the rotifers were alive. The experiment is ongoing and the next rehydration is scheduled for the eighth anniversary in 2023.
 Figure 15. This shell of a sunflower seed was from a dry birdbath. The piles of red particles encrusting it were dry rotifers (ruler in millimetres). Upon placement in water, the majority of the rotifers revived.
Figure 15. This shell of a sunflower seed was from a dry birdbath. The piles of red particles encrusting it were dry rotifers (ruler in millimetres). Upon placement in water, the majority of the rotifers revived.
Bdelloids often share their habitats with tardigrades and nematodes, which owe their ability to survive drying at least partially to the accumulation of high concentrations of the carbohydrate trehalose in their tissues. Bdelloids, however, lack trehalose and how they achieve their drying tolerance is not known (Lapinski & Tunnacliffe, 2003)[12]. Once again, bdelloids are anything but ordinary.
How to study and identify bdelloids
Bdelloid species are notoriously difficult to identify. The main problem is that many species, including some supposedly common and cosmopolitan species, for example, Adineta vaga and Philodina roseola, were originally described so inadequately that they cannot be identified unequivocally any more (Örstan, 2020b)[19]. For example, the morphology of the upper lip of the specimen tentatively identified as Macrotrachela habita in Fig. 6A does not exactly match the morphology of the upper lip in Bryce’s (1894)[2] drawing of the species (Fig. 6B). Was Bryce’s drawing inaccurate or is the specimen in Fig. 6A a different species? Moreover, genetic analyses have shown that some traditional species are actually species complexes consisting of cryptic taxa that differ genetically but appear to be identical morphologically (see for example, Fontaneto et al., 2009)[7]. Very careful examinations of the members of such species complexes are necessary to determine whether or not they differ from each other morphologically.
There are also practical difficulties in the study of bdelloids. As mentioned in the Introduction, to distinguish morphovariants from each other it is often necessary to examine minute morphological details of live and often unruly animals at high magnifications. To make matters worse, a readily available anesthetic effective for all species is not available. Some success has been reported with bupivacaine, a local anesthetic used for various medical procedures. However, bupivacaine may be difficult to obtain by amateur microscopists. Moreover, until recently, there was no procedure to preserve the extended bodies of bdelloids without damage or distortion. Killing of bdelloids with boiling water – recommended in some publications – should be avoided, because hot water literally cooks the animals resulting in distorted and damaged bodies and coagulated internal organs. Instead, I advocate the use of hot glutaraldehyde (about 2.5%), which preserves bdelloids in a superior condition suitable for both identification purposes and anatomical studies (Örstan, 2015)[16]. One should also avoid the use of a glass pipet to transfer active bdelloids (unless a large number is being transferred). This is because the bdelloids are likely to remain attached to the inside of the pipet after the pipet is emptied. Instead, a microloop or a microspatula, constructed from minuten insect pins, should be used (Fig. 16).
 Figure 16. Tools useful for the study of bdelloids: microloop, microspatula and microprobe. They were made from the finest minuten insect pins. Two were glued to old pens and one was held by a pin vise.
Figure 16. Tools useful for the study of bdelloids: microloop, microspatula and microprobe. They were made from the finest minuten insect pins. Two were glued to old pens and one was held by a pin vise.
If available, the use of a microscope with differential interference contrast (DIC) is recommended. I also emphasize that high-resolution microphotography is essential to develop reliable descriptions of bdelloids. It is usually difficult to visually examine and measure live and active specimens. But if many photographs of a specimen are taken at high magnifications, selected photographs not only will show the morphological traits and create permanent records of them, but will also allow for their measurements. Some photographs may also reveal details that may have been missed under the microscope. High-resolution videos are also useful in the study of the behaviours of bdelloids. For example, a video was included in the description of Coronistomus impossibilis to highlight not just its morphology but also its behavior (see below). When necessary, informative frames may be extracted from videos (Figs. 6A, 11).
Coronistomus impossibilis, a new fluvial bdelloid rotifer
The examination of a newly isolated bdelloid morphovariant should start with a cursory examination of its overall morphology under a stereomicroscope or at the low powers of a compound microscope and then continue with the detailed examination of the morphology at a higher magnification, usually at 200× or 400×. In recent papers, I discussed the taxonomically significant morphological traits of bdelloid rotifers in the families Adinetidae (Örstan, 2018)[17] and the Philodinidae (Örstan, 2020a)[18]. The discussions in those papers may be generalized to include the remaining families of bdelloids. To fully characterize a bdelloid morphovariant one should take into account all of the traits mentioned in those papers. Any deviation from the usual morphology and consistent idiosyncrasies in behavior observed in the specimens would be significant and should be documented. At the same time, potential intraspecific variability of morphological traits should be taken into account.
It is often necessary to know the number of toes of a specimen to determine its genus. The toes are best seen and counted, especially if they are short, when a bdelloid is creeping on the underside of a cover glass (Fig. 11). Sometimes they attach to the cover glass on their own. Otherwise, one may place a specimen in a small drop of medium on a cover glass, wait for it to attach its toes to the glass, then quickly turn the cover glass upside down and place it on microscope slide. The rotifer may crawl on the underside of the cover glass long enough for the microscopist to examine its toes. Some species in the three-toed genera Macrotrachela (Philodinidae) and Habrotrocha (Habrotrochidae) look alike and can be placed in the correct genus only by the examination of their stomachs for a lumen. The stomach contents of the species that have a lumen usually have a fine, granular appearance, while those of the species in the lumenless Habrotrochidae form large globules (pellets) that retain their form for a short while outside the body (Fig. 17). So the observation of a rotifer voiding its intestine may help determine its taxonomic placement!
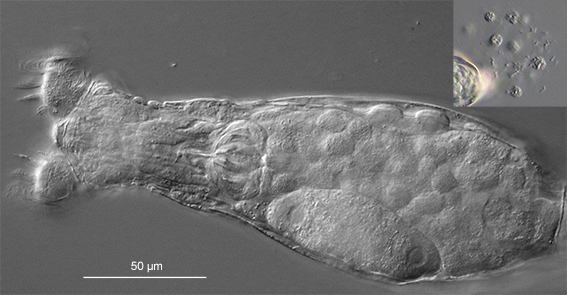 Figure 17: A feeding Habrotrocha. The large pellets filling its stomach retained their shapes after they were excreted (inset; not to scale) (DIC).
Figure 17: A feeding Habrotrocha. The large pellets filling its stomach retained their shapes after they were excreted (inset; not to scale) (DIC).
The corona and the upper lip can be seen only when a bdelloid is feeding. The morphology of the upper lip is an especially important taxonomic trait necessary for the identification of many species (Figs. 5A, 6). Under the cover glass, some bdelloids feed readily and steadily for long periods, while others swim continuously, which makes it hard to examine them and still others are frustratingly stubborn to open their coronas. When a rotifer is not feeding there isn’t much one can do other than wait patiently. Other taxonomically significant traits are spines or appendages that may be present on various parts of the body (Fig. 4) and the eye spots (Fig. 10) that may be on the brain (Philodina, Abrochtha, Dissotrocha, etc.) or on the rostrum (Rotaria, etc.) and the length of the antenna. The length and the shape of the spurs should also be noted as they are often diagnostic.
The dimensions of the trophi should be measured and the number of major teeth should be counted. These procedures are easiest to carry out on the photographs of the trophi. The trophi may be photographed in live specimens that are slightly compressed under the cover glass. If the cover glass is fixed in place with dabs of petroleum jelly at its corners, even an oil immersion objective may be used to photograph the trophi and the other features of a compressed live rotifer. It is also possible to extract the trophi by the use of household bleach, but this is a tricky operation, because it is very easy to lose the trophi once it is free of tissues (De Smet, 1998)[4].
The foundations of the current taxonomy of bdelloids dates back to Bryce’s (1910)[3] paper in the Journal of the Quekett Microscopical Club. That work, now more than a century old, is still informative to read. A more recent key to the genera was published in the Quekett Journal of Microscopy (Turner, 1999)[23]. Donner’s 1965 book Ordnung Bdelloidea[5] is the most complete identification guide for bdelloid species, although it should be used with care not only because some of Donner’s taxonomic opinions are questionable and outdated, but also because new species have been described since then. The last review of bdelloid species added more than 40 taxa that had been described since Donner’s book (Örstan & Plewka, 2017)[22] and 11 additional species have been described since then. Clearly, the work of the rotiferologist is not yet finished.
References
1. Baker, H. 1764. Employment for the Microscope. http://www.biodiversitylibrary.org/item/99340#page/7/mode/1up
2. Bryce, D. 1894. Further notes on macrotrachelous Callidinae. Journal of the Quekett Microscopical Club 5: 436-455
3. Bryce, D. 1910. On a new classification of the Bdelloid Rotifera. Journal of the Quekett Microscopical Club 11: 61–92
4. De Smet, W. H. 1998. Preparation of rotifer trophi for light and scanning electron microscopy. Hydrobiologia 387/388: 117–121
5. Donner, J. 1965. Ordnung Bdelloidea. Akademie-Verlag.
6. Fontaneto, D., Kaya, M., Herniou, E. A., Barraclough, T. G. 2009. Extreme levels of hidden diversity in microscopic animals (Rotifera) revealed by DNA taxonomy. Molecular Phylogenetics and Evolution 53: 182–189
7. Fontaneto, D., De Smet W. 2015. Rotifera in A. Schmidt-Rhaesa (ed.) Handbook of Zoology, Gastrotricha, Cycloneuralia and Gnathifera, 3: Gastrotricha and Gnathifera, pp. 217–300. De Gruyter
8. Ford, B. J. 1982. The Rotifera of Antony van Leeuwenhoek. Microscopy 34: 362–373
9. Hejnol, A. 2015. Gnathifera in A. Wanninger (ed.) Evolutionary Developmental Biology of Invertebrates 2: Lophotrochozoa (Spiralia), pp. 1–12. Springer-Verlag
10. Iakovenko, N. S., Smykla, J., Convey, P., Kašparová, E., Kozeretska, I. A., Trokhymets, V., Dykyy, V. I., Plewka, M., Devetter, M., Duriš, Z., Janko, K. 2015. Antarctic bdelloid rotifers: diversity, endemism and evolution. Hydrobiologia 761: 5–43
11. Laine, V. N., Sackton, T., Meselson, M. 2020. Sexual Reproduction in Bdelloid Rotifers; bioRxiv 2020.08.06.239590
12. Lapinski, J., Tunnacliffe A. 2003. Anhydrobiosis without trehalose in bdelloid rotifers. FEBS Letters 553: 387–390
13. Melone, G., Fontaneto D. 2005. Trophi structure in bdelloid rotifers. Hydrobiologia 546: 197–202
14. Murray, J. 1910. Antarctic Rotifera. British Antarctic Expedition 1907–9, Reports on the Scientific Investigations 1: 41–65
15. Örstan, A. 1998. Factors affecting long-term survival of dry bdelloid rotifers: a preliminary study. Hydrobiologia 387/388: 327–331
16. Örstan, A. 2015. A method for the preservation of bdelloid rotifers for taxonomical and anatomical studies. Quekett Journal of Microscopy 42: 355–359
17. Örstan, A. 2018. Taxonomic morphology of the genus Adineta (Rotifera: Bdelloidea: Adinetidae) with a new species from a suburban garden. Zootaxa 4524: 187–199
18. Örstan, A. 2020a. Taxonomical characterization of bdelloid rotifers in the family Philodinidae. Quekett Journal of Microscopy 43, 611–620
19. Örstan, A. 2020b. The trouble with Adineta vaga (Davis, 1873): a common rotifer that cannot be identified (Rotifera: Bdelloidea: Adinetidae). Zootaxa 4830: 597–600
20. Örstan, A. 2020c. Birdbath rotifers: masters of survival. Quekett Bulletin No. 79: 14–16
21. Örstan, A. 2021. An extraordinary new fluvial bdelloid rotifer, Coronistomus impossibilis gen. nov., sp. nov., with adaptations for turbulent flow (Rotifera: Bdelloidea: Coronistomidae fam. nov.). Zootaxa 4966: 016–028
22. Örstan, A., Plewka, M. 2017. Bdelloid rotifer taxa described since 1965 with the addition of a new species. Quekett Journal of Microscopy 43: 31–37
23. Turner, P. 1999. A simple generic key to bdelloid rotifers. Quekett Journal of Microscopy 38: 351–356
24. Wesenberg-Lund, C. 1930. Contributions to the Biology of the Rotifera, Part II: The Periodicity and Sexual Periods, p. 186. A. F. Host and Son, Copenhagen

This work (excluding Figure 1B, Figure 6B, Figure 13) is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

