Webmaster’s blog 2016
Alan Wood’s microscopical diary
December 2016
Soon after I bought my Olympus BH-2 back in 2003, I bought a BH-RLA Brightfield/Darkfield Reflected Light Illuminator with a set of Neo 5×, 10×, 20× and 40× objectives and found that I couldn’t raise the stage quite high enough to focus with these short-barrel objectives. I didn’t have a BHT instruction manual at that time. For a while, I just used a couple of blank slides to raise the specimens a little, until I noticed a screw near the condenser height adjustment knob. A 3 mm Allen key fitted, so I took a chance, carefully loosened the screw and found that I could raise the whole stage+condenser assembly and re-tighten the screw – problem solved. The screw is indicated with a yellow arrow in this photograph:
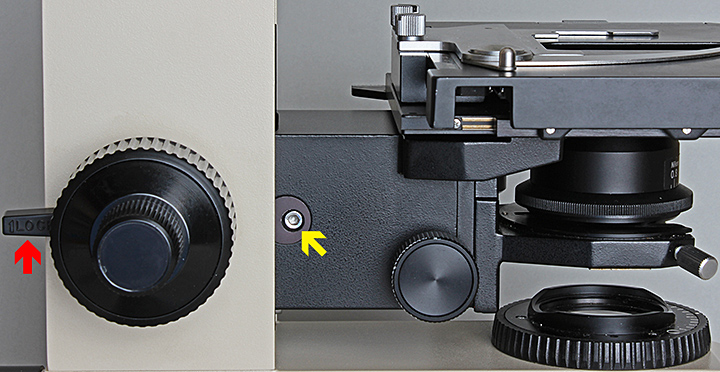 Stage block locking screw (yellow arrow) and pre-focusing lever (red arrow)
Stage block locking screw (yellow arrow) and pre-focusing lever (red arrow)
Another thing to check if you can’t raise the stage enough is that the pre-focusing lever (red arrow in the photo above) is disengaged by pushing it to the back.
Recently I tried to photograph the top surface of a 25 mm high cylinder for my report on the Arkwright Scholars Workshop, and I had the opposite problem – I couldn’t lower the stage enough. This time I knew what to do; behind the stage+condenser assembly there is a set screw that can be put in either of two tapped sockets. The instruction book says you can get access to this screw by temporarily raising the assembly, but the screw would not budge with my 3 mm Allen key. Fortunately, I knew that the whole assembly could be removed (remove the condenser, nosepiece and stage first) by lowering the stage, loosening the locking screw, sliding the assembly upwards and then tilting it downwards, to improve access to the set screw:
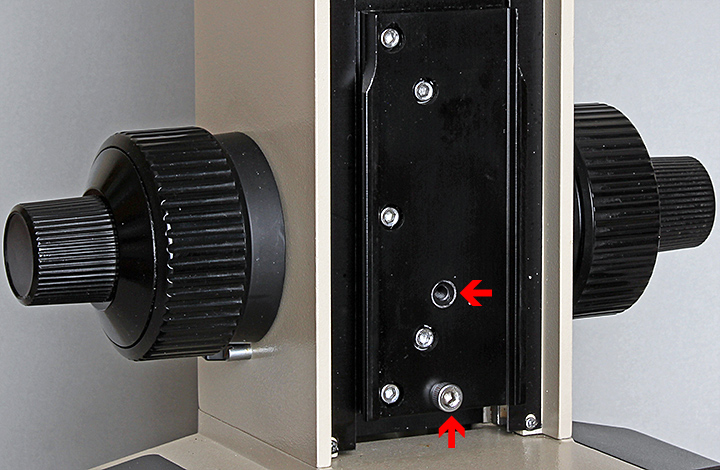 Set screw and alternative socket for height of stage block
Set screw and alternative socket for height of stage block
I still couldn’t move the set screw with my 3 mm Allen key, but the outside of its head is knurled and I eventually managed to grip it and loosen it with a mole wrench. Then all I had to do was put the set screw in the lower socket and re-assemble everything. Out of curiosity, I measured the maximum gape (distance between stage and nosepiece) and it is approximately 96 mm, so with a long-barrel objective (45 mm parfocal) it should just be possible to focus on the top of a specimen that is 50 mm tall.
 Gape adjusted to allow a 25 mm tall specimen to be photographed
Gape adjusted to allow a 25 mm tall specimen to be photographed
Here is what I was trying to photograph:
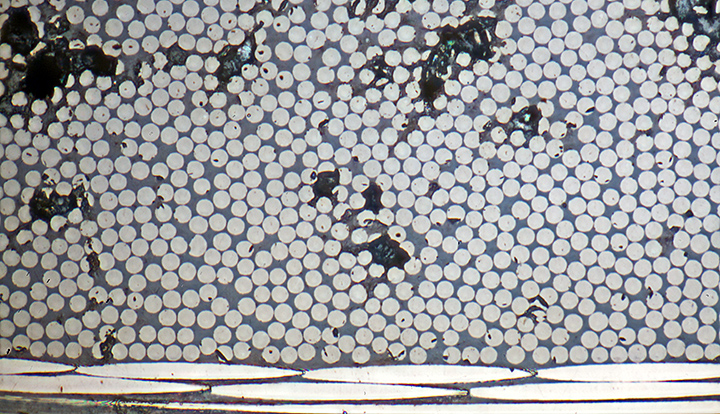 Sections through carbon fibres (7 µm diameter) in laminate
Sections through carbon fibres (7 µm diameter) in laminate
Olympus MSPlan 50× objective, NFK 2.5× photo eyepiece, 11 images at 1 µm intervals combined in Zerene Stacker
When the Club’s website was being re-created in WordPress, we reluctantly decided to abandon the forum because it was no longer being used by Quekett members. Now we are trying a different way to allow members to communicate online, a Facebook group.
You need to be signed in to Facebook, then go to the group page and click “Join group”. If you are not on Facebook, contact the Webmaster and I will send you an e-mail address and password that will enable you to have a look.
 QuekettMicro group on Facebook
QuekettMicro group on Facebook
November 2016
At the end of October, I made a trap to take part in Chris Thomas’s survey of the invasive fruit-fly Drosophila suzukii (Matsumura) and caught a few in the back garden.
 Trap for fruit-flies, using apple cider vinegar to attract them
Trap for fruit-flies, using apple cider vinegar to attract them
After rinsing to get rid of the smell of apple cider vinegar the flies looked clean so I took some photographs.
 Female of Drosophila suzukii
Female of Drosophila suzukii
The fly is about 2.8mm long. Canon EOS 600D, Zuiko Auto-Macro Lens 38mm f/2.8 at f/4 on Telescopic Auto Tube 65–116, magnification ×4, stack of 83 images at 40µm intervals combined in Zerene Stacker, lit by OTT-LITE TrueColor 13w task light (no diffuser)
Unlike other species of Drosophila, D. suzukii has a serrated ovipositor that allow the female to cut into undamaged fruit and lay her eggs inside. The larvae then have plenty of food and are protected from predators and parasitoids.
 Serrated ovipositor of Drosophila suzukii
Serrated ovipositor of Drosophila suzukii
Cropped from the photo above
At the East of England meeting last month, Dave Skeet told us about programs that can convert a series of still images into a video. I first tried Photolapse 3, but it wouldn’t load the first image and it displaced the second image to one side. Then I tried Windows Movie Maker, which worked as expected and allowed me to specify the time for which each image is displayed. My subject was stacking, using the image of D. suzukii as an example.
Click the arrow at bottom left to watch the video (1 minute), click the symbol to the left of “vimeo” for full screen.
Here is another video, this time of Zerene Stacker in action, combining 83 images of D. suzukii into a single image with extended depth of field. The video was recorded with the free OBS Studio software.
Click the arrow at bottom left to watch the video (4 minutes), click the symbol to the left of “vimeo” for full screen.
I showed the above videos and photos on the Quekett stand at the annual exhibition of the British Entomological and Natural History Society on Saturday 12th November, together with my recently-acquired Olympus BHMJ (formerly owned by Brian Bracegirdle) kitted out with a trinocular head and my EOS 5D Mark II. The stand attracted so many visitors with all sorts of questions about microscopes that I ran out of handouts and business cards. We hope to see some of the visitors at Quekett meetings in the near future.
 Trinocular Olympus BHMJ and Zerene Stacker
Trinocular Olympus BHMJ and Zerene Stacker
October 2016
A very busy month, with 6 events to attend and report on. Saturday 1st October was the Annual Exhibition of Microscopy, and I took 2 heavy bags with my trinocular Olympus CH-2 microscope, Canon EOS 5D Mark II camera and Lenovo laptop for a comparison between a conventional microscope and the ioLight portable microscope. The ioLight produced creditable images, but not as good as the Olympus (compare the images).
Saturday 8th October was the Langton Matravers meeting. Joan Bingley kindly gave me a lift again, and I took the same equipment as Quekex, but this time to show how easy it is to take good quality digital photomicrographs.
 Complete kit for taking photomicrographs
Complete kit for taking photomicrographs
Kai came to Langton too, and was fascinated by Klaus Kemp’s diatom arrangements. Klaus explained how he makes the glass needles that are so fine they are hard to see, showed her how he manipulates a diatom, and let her try it for herself.
 Kai watching Klaus Kemp manipulating a diatom
Kai watching Klaus Kemp manipulating a diatom
Saturday 15th October was Microscopium at St Albans, always an interesting event even if you don’t take advantage of any of the bargains. Lots of microscopes to try out, accessories, slides and books to browse through, and microscopists from far and wide to chat to.
Sunday 23rd October was the Antique Scientific Instrument Fair in London, where Stephen Parker and I manned the Quekett stand. This time there were some oil lamps for microscopes; might be useful at those venues that insist on PAT testing electrical equipment to which it doesn’t apply.
 Newton (left) and Beck oil lamps for microscopes
Newton (left) and Beck oil lamps for microscopes
On Friday 28th October I was one of the Quekett team at the National Honey Show, where Norman Chapman showed how he produces his drawings of pollen grains on tracing paper over a printed photomicrograph.
 Norman Chapman drawing a pollen grain
Norman Chapman drawing a pollen grain
Saturday 29th October was the East of England meeting, miles from anywhere but Joan Bingley gave me a lift there and Robert Ratford took me back to London. Some interesting exhibits, but the highlight was Stephen Livermore’s talk on preparing wax cells for fluid mounts. He demonstrated his techniques and told us the details of his formulae. Read Stephen’s notes on his techniques and formulae
 Stephen Livermore making a wax cell
Stephen Livermore making a wax cell
September 2016
I missed all of the Quekett events this month because we were back in Thailand for the ceremony to mark 100 days since my mother-in-law died. We also caught up with lots of friends, which often involved meals in restaurants that would be great places for a Quekett excursion, with freshly-cooked food available all day in locations with their own ponds or even their own rice field and plenty of parking space.
 “Year of the Rabbit” restaurant near Ratchaburi
“Year of the Rabbit” restaurant near Ratchaburi
Back to the UK and reality on Friday 30th, helping to set up for Quekex in the afternoon, then preparing my exhibit and notes in the evening.
August 2016
On Tuesday 9th August, there was a really good gossip meeting on “Objects for the stereomicroscope”. I took my Olympus SZ4045 with an Ikea Jansjö lamp and some specimens from the garden that can be observed with no preparation: spore-containing sori on the underside of a frond from an ornamental fern, seed cones of Lawson’s cypress (Chamaecyparis lawsoniana) and grey foliose lichen on a twig from a pear tree.
 Spore-containing sori on the underside of a frond from an ornamental fern
Spore-containing sori on the underside of a frond from an ornamental fern
Canon EOS 600D, Zuiko Auto-Macro Lens 38mm f/2.8 at f/4 on Telescopic Auto Tube 65–116, magnification ×3.1, stack of 31 images in Zerene Stacker, lit by 2 compact fluorescent lamps with a Kleenex diffuser
 Grey foliose lichen (? Hypogymnia sp.) on a pear twig
Grey foliose lichen (? Hypogymnia sp.) on a pear twig
Canon EOS 600D with 60 mm EF/S macro lens at f/4.5, stack of 18 images in Zerene Stacker, lit by 2 compact fluorescent lamps with a Kleenex diffuser, field width 20 mm
I also took a dark-ground attachment and some slides, and David Linstead photographed one of them with an afocal system consisting of a Sony NEX 5N mirrorless compact system camera with a Minolta 50 mm lens and a Scopetronix MaxView Plus wide-field 6×30 eyepiece. When I got home, I photographed the same slide with the same dark-ground attachment but using a camera and a macro lens.
 Zoea larva of porcelain crab (Porcellana platycheles) [by David Linstead]
Zoea larva of porcelain crab (Porcellana platycheles) [by David Linstead]
Olympus SZ4045 stereomicroscope, stitch of 3 images
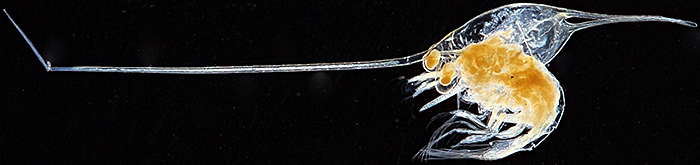 Zoea larva of porcelain crab (Porcellana platycheles)
Zoea larva of porcelain crab (Porcellana platycheles)
Canon EOS 600D with 60 mm EF/S macro lens at f/4.5, stack of 25 images in Zerene Stacker, field width 9 mm
On Saturday 20th August, Kai and I went on the Quekett excursion to Warnham Local Nature Reserve, the first time Kai has been there. The weather was bad, but it didn’t take long to collect material from the dipping ponds and bring it back to examine with our trinocular Olympus SZ4045 stereomicroscope in the comfort of the Visitor Centre. Graham Matthews’ wife (Noi) was there too, so they had a good natter in Thai.
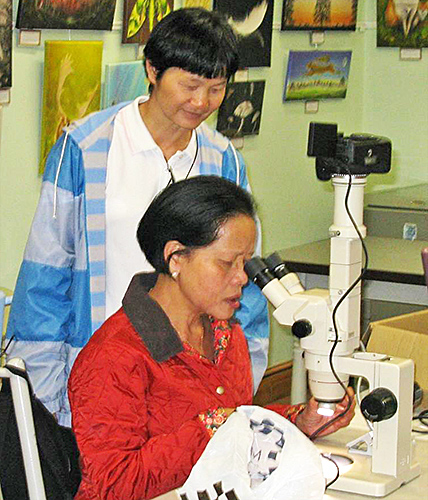 Kai and Noi observing a budding green hydra
Kai and Noi observing a budding green hydra
July 2016
I obtained lots of Olympus microscope brochures and price lists from Peter Evennett at Penkridge, so I took some of them to the gossip meeting on “My latest microscopical acquisition” on Tuesday 12th July. They are all available as free PDFs on my Olympus microscope documentation web page.
Kai can’t often get to meetings, but she was able to come on the Basingstoke Canal excursion on Saturday 16th July and we had a good day collecting and observing.
 Kai with Maurice Moss and Mary Morris
Kai with Maurice Moss and Mary Morris
We took the Olympus CH-2 with the dual-observer attachment so that it was easy for me to show her things.
 Olympus CH-2 with dual observer attachment
Olympus CH-2 with dual observer attachment
We spent a few days in St Mawes in Cornwall, including walks along the shore. There were lots of rock pools with seaweed, limpets, barnacles and winkles, but none of the small crabs and shrimps that I was expecting to see.
 Rock pool at St Mawes
Rock pool at St Mawes
June 2016
After spending most of May living in a Thai hospital looking after my mother-in-law, we were ready for a break and spent a few days in Morecambe (where I lived while at university) and the Lake District. The sand in Morecambe contains lots of different materials, mostly mineral in origin.
 Sand from Morecambe
Sand from Morecambe
Canon EOS 600D, 60 mm EF/S macro lens at f/4.5 with 8-dioptre close-up lens, stack of 23 images in Zerene Stacker, lit by 2 compact fluorescent lamps with a Kleenex diffuser, field width 15 mm
I missed all the Quekett meetings this month because we had to rush back to Thailand for my mother-in-law’s funeral.
May 2016
My websites on Olympus OM macro equipment and Olympus microscopes lead to some interesting e-mails. Some people get the impression that I am a dealer, which I am definitely not, but I can sometimes tell people where to find the items they are looking for.
Recently I have been asked where to find an Olympus U-POT polariser. This is still available new from Olympus (but apparently it costs over £200) and it sometimes appears on eBay. It is marked “U-POT” so it should be easy to locate. An alternative is the A-PO polariser that was available for the BH, CH, BH-2 and CH-2 microscopes; it is exactly the same as the U-POT but is not marked with its model, and so it can sometimes be found at reasonable prices from eBay sellers who don’t know what they have. It took me a few years to find one, but I paid under £25 for a “gray filter”, including postage from the USA.
 Olympus A-PO polariser
Olympus A-PO polariser
The U-POT and A-PO fit into the light outlet on the base of the microscope and are easy to rotate to achieve maximum extinction. However, it is also easy to use a polarising filter intended for a camera. A Hoya 55 mm filter fits neatly on top of the light outlet on my BH-2.
 Hoya 55 mm polarising filter on Olympus BH-2
Hoya 55 mm polarising filter on Olympus BH-2
A linear polarising filter will work either way up. Circular polarising filters are more common and consist of a linear filter plus a ¼ λ wave plate; one way up they work just like a linear filter, the other way up they introduce different colours.
Another item I have recently been asked to help find is the VST-E Extension Bar for the VST-1 Macrophoto Stand, part of the macro equipment for Olympus OM 35 mm cameras. The VST-E screws into the top of the column of the stand, to provide a greater working distance.
 Olympus OM Macrophoto Stand Extension Bar VST-E
Olympus OM Macrophoto Stand Extension Bar VST-E
Unfortunately, the VST-E has no markings at all, so when it is separated from the stand not many people will know what it is, and you would very lucky to find one for sale on its own. I think it should be fairly easy to make one of these on a lathe, and I am surprised that nobody seems to have made some to sell.
April 2016
Most of the photomicrographs and macro photographs in my blog were made using focus stacking software, but I have never previously included a comparison of a stack and a single image. For someone like me who used to use 35mm film, the difference in depth of field is amazing.
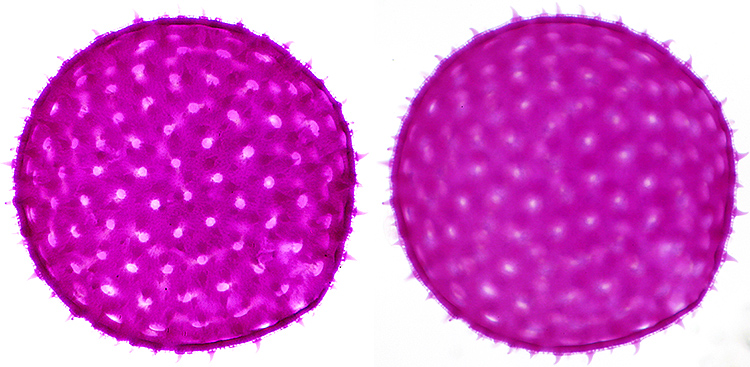 Stained single grain of pollen of mallow (Malva sp.) from an NBS slide, diameter 150 µm, Olympus SPlan 40× objective, NFK 2.5× photo eyepiece
Stained single grain of pollen of mallow (Malva sp.) from an NBS slide, diameter 150 µm, Olympus SPlan 40× objective, NFK 2.5× photo eyepiece
Stack of 12 images in Zerene Stacker (left) and single image (right)
Joan Bingley kindly gave me a lift to the Langton Matravers meeting in Dorset on Saturday 9th April, the first time I have been there. After reading lots of meeting reports, it was good to finally meet Brian Darnton with his forams, Grenham Ireland with his tardigrades, Jack Cohen with his amoebae and Derek Stevens with his pond life.
Just before the M. C. Cooke Lecture on Tuesday 12th April, Carel Sartory presented me with a Certificate of Honorary Membership in recognition of all the work I put into rescuing, restoring and improving the Club’s website after Cubik Solutions dropped support and then went into liquidation.
 Carel Sartory presenting my Certificate of Honorary Membership [photo by Chris Thomas]
Carel Sartory presenting my Certificate of Honorary Membership [photo by Chris Thomas]
On Saturday 16th April, I went to the Penkridge meeting in Staffordshire with Joan. Peter Evennett and Chris Hammond had lots of interesting items but I confined myself to an instruction book for my Olympus CHS microscope. Mike Smith was selling slides that he had prepared, including whole tardigrades, fleas and Obelia, and sections of flowers, slugs and earthworms, and I bought a few.
 Slide of Obelia
Slide of Obelia
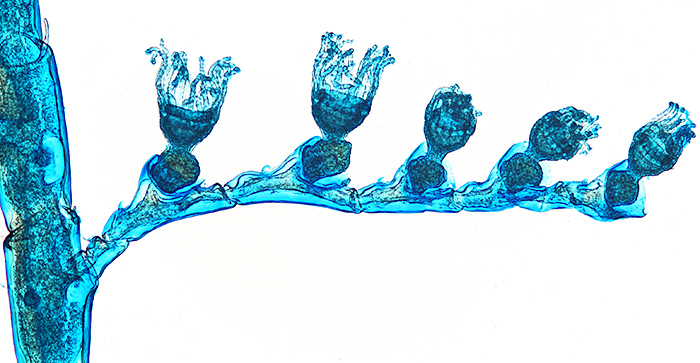 Obelia, stained with Alcian blue, slide by Mike Smith
Obelia, stained with Alcian blue, slide by Mike Smith
Olympus SPlan 4× objective, NFK 2.5× photo eyepiece, 14 images at 10 µm intervals combined in Zerene Stacker
 Slide of daffodil ovary
Slide of daffodil ovary
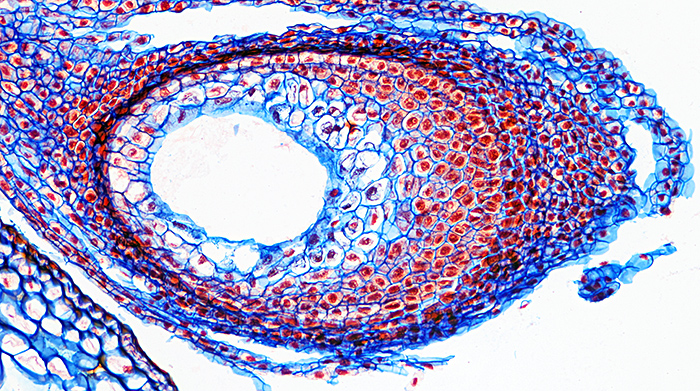 Daffodil ovary, stained with Astra blue and safranin, slide by Mike Smith
Daffodil ovary, stained with Astra blue and safranin, slide by Mike Smith
Olympus SPlan 10× objective, NFK 2.5× photo eyepiece, 12 images at 2 µm intervals combined in Zerene Stacker
 Slide of earthworm (Lumbricus terrestris)
Slide of earthworm (Lumbricus terrestris)
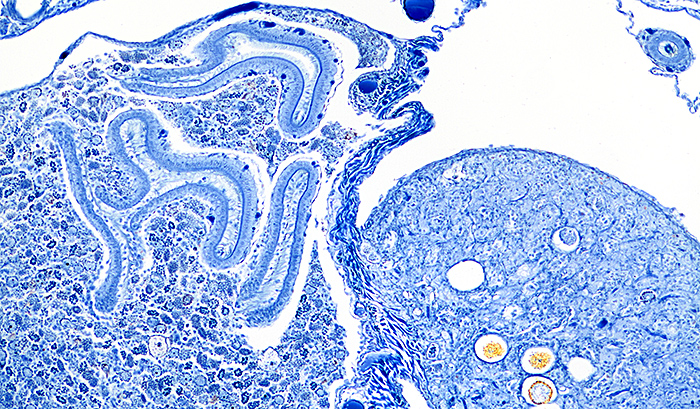 Lumbricus terrestris, stained with Mallory’s triple stain, slide by Mike Smith
Lumbricus terrestris, stained with Mallory’s triple stain, slide by Mike Smith
Olympus SPlan 10× objective, NFK 2.5× photo eyepiece, 12 images at 2 µm intervals combined in Zerene Stacker
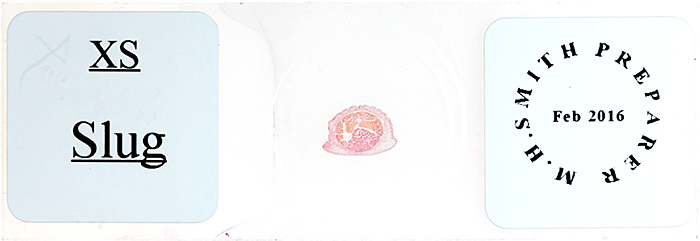 Slide of slug
Slide of slug
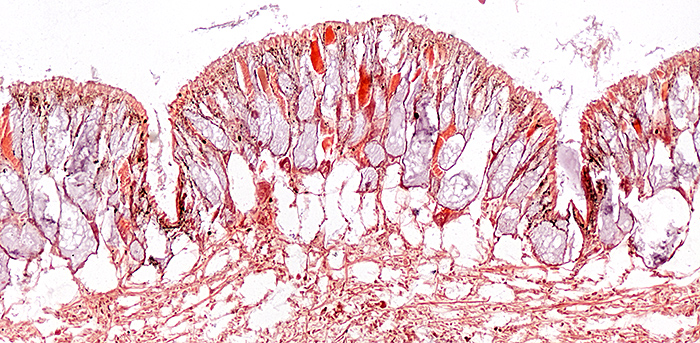 Slug, stained with haematoxylin and Biebrich scarlet, slide by Mike Smith
Slug, stained with haematoxylin and Biebrich scarlet, slide by Mike Smith
Olympus SPlan 10× objective, NFK 2.5× photo eyepiece, 11 images at 2 µm intervals combined in Zerene Stacker
March 2016
I am managing to reduce the time I spend on web development and increase the time I spend on microscopy, and on Saturday 5th March I went to the South Thames Discussion Group at its new home in Wallington. I took a selection of commercial and home-made cup stages and ball tables that are useful for orienting specimens under a stereomicroscope or a camera, and also a pair of goniometers that do the same job but with much greater precision and a much higher cost. I provided artificial butterflies of different sizes printed on pink card so that visitors could try out the gadgets.
 Ball tables and a cup stage with cork or Plastazote inserts for pins, or a crocodile clip
Ball tables and a cup stage with cork or Plastazote inserts for pins, or a crocodile clip
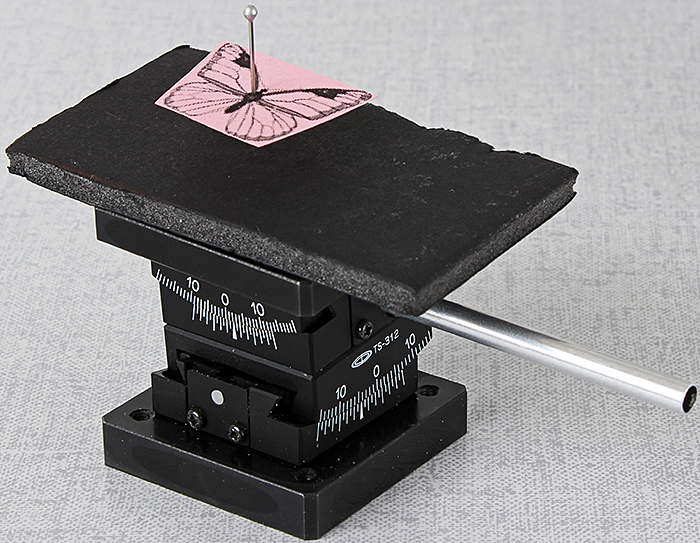 Stacked Chuo goniometers
Stacked Chuo goniometers
The following Saturday was the Reading Convention, where Tony Pattinson demonstrated his tank cam with a small container of pond water containing copepods and bryozoans, with a live video feed on his laptop.
I thought I was getting a break from producing web pages, but I was persuaded that we should contribute to British Science Week 2016 with a set of 10 pages on members’ interests. Fortunately I didn’t have to write them all because members including David Linstead and Tony Pattinson contributed some really good articles.
February 2016
Now that the Club has regained use of the Dorothea Bate Room for meetings, all of the microscopes have had to be carried back from the temporary store rooms. David Linstead, Dennis Fullwood, Paul Smith and Jacky McPherson have done a great job, even adjusting the spacing of the shelf units so that the microscopes fit better. I joined them on Thursday 4th February to lend a hand and to experiment with the Olympus BHC that is fitted out for Hoffman Modulation Contrast. I used a phase telescope to try to set it up properly, but failed to get anything that looked different from normal bright field illumination. I have since found out what I did wrong; I should have used a substage polariser.
I had another meeting with Jellybean Creative Solutions on Wednesday 3rd February to review the few remaining problems with the new website. Now it is Friday 5th February, and I think all of the bugs have been fixed, so it is time to ask the other members of the committee to have a last check before I accept the website. Monday 8th February, nobody found any problems with the site design so I have formally accepted it, subject to fixing of a problem with logging in. Thursday 11th February, the logging-in problem has been fixed. Friday 12th February, implemented a back-up strategy for the website and the associated database; the backup files are available to us (unlike the Cubik ones), independent of the hosting company, and should allow us to restore the complete website in hours or days. Monday 15th February, the new website is up and running. Now I can start catching up with all the things I have had to put on hold for the last few months.
Saturday 20th February was the workshop on photography through the microscope in the Natural History Museum. I used the Club’s Olympus BHC microscope (with Hoffman Modulation Contrast set up properly), and took in my BH-TR30 trinocular head, PM-ADF eyepiece adapter, FK 2.5× photo eyepiece, Photomicro Adapter L, OM-EOS adapter and Canon EOS 5D Mark II digital SLR to demonstrate eyepiece projection.
 Canon EOS 5D Mark II digital SLR on a trinocular Olympus BH compound microscope
Canon EOS 5D Mark II digital SLR on a trinocular Olympus BH compound microscope
I have quite an assortment of Olympus accessories, so I was also able to show all the bits and pieces separately.
 Olympus adapters for connecting cameras to microscopes
Olympus adapters for connecting cameras to microscopes
You can find out more about this equipment on my website: Olympus microscopes and photomicrographic equipment.
January 2016
I have been so busy with the new website that I only used a microscope once this month. My wife bought a top-up card for her mobile phone, scratched it gently to reveal the code, but then couldn’t top up on the phone or on the website. I hate mobile phones, but I had a go and noticed that the website said the code should be 14 characters, but the code on the card was only 13 characters. I had a look through my Olympus SZ4045 stereomicroscope and I could see that there were 2 very thin layers covering the code, one blue and one silver, and that the lower silver layer had not been completely removed. With a bit of careful scratching, the 14th character was revealed and the phone could be topped up.
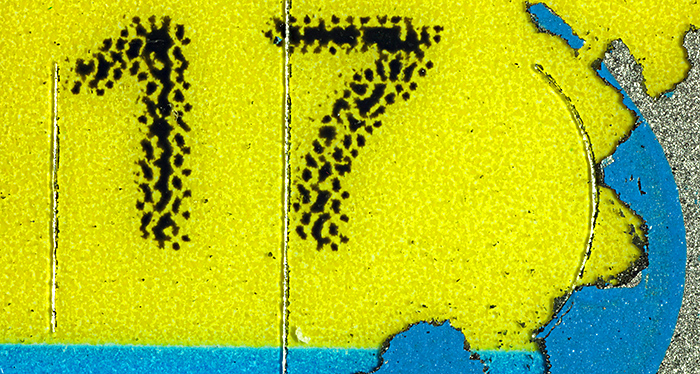 Lebara top-up scratch card: the numbers are 2.3 mm tall
Lebara top-up scratch card: the numbers are 2.3 mm tall
Canon EOS 5D Mark II, Olympus Zuiko Auto-Macro 38 mm at f/4 on Telescopic Auto Extension Tube 65~116, magnification ×4.4, 20 images at 0.04 mm intervals combined in Zerene Stacker
Definitely a good result: my wife is happy that she can use her phone, and she had to admit that my expensive stereomicroscope had come in useful.
Commenting on this blog
If you would like to comment on anything in this blog, please contact Alan Wood.
About this blog
I am Alan Wood, former webmaster for the Quekett website, and author of several web pages on Olympus microscopes. I spend too much time writing about microscopes and buying more equipment. I hope this blog will help me to focus on using my microscopes so that I have something to write about!

