Barnard Awards 2023 (Technical)
The judge, Tom Hartman, considered the photographs before they were shown at the Annual Exhibition of Microscopy at Potters Bar on Saturday 14th October 2023, and had the difficult job of deciding which of the ones from members of the Quekett, the Iceni Microscopy Study Group and the Postal Microscopical Society were of a sufficiently high standard to deserve a certificate.
He awarded certificates to Thomas Consi (Stentor dividing), Jonathan Crowther (Actinoptychus heliopelta), David Furness (Field of cilia), Pam Hamer (Copperas ore) and Mike Smith (L.S. honeybee antenna).
Quekett members can see larger images and the judge’s comments in the Photography showcase.
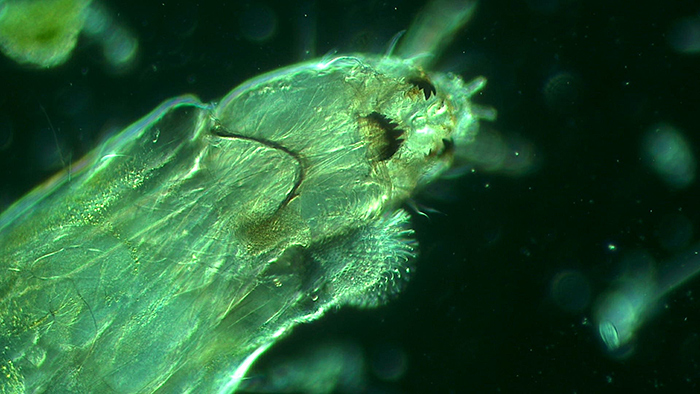 Alan Jones: Hairy worm
Alan Jones: Hairy worm
Sample taken from home aquarium for cold-water fish. Wet mount with coverslip.
Brunel SP500 trinocular, objective 20×, HDMI, USB camera.
Green ground achieved by placing a home-made green central stop on top of the condenser lens and below the flip-top lens.
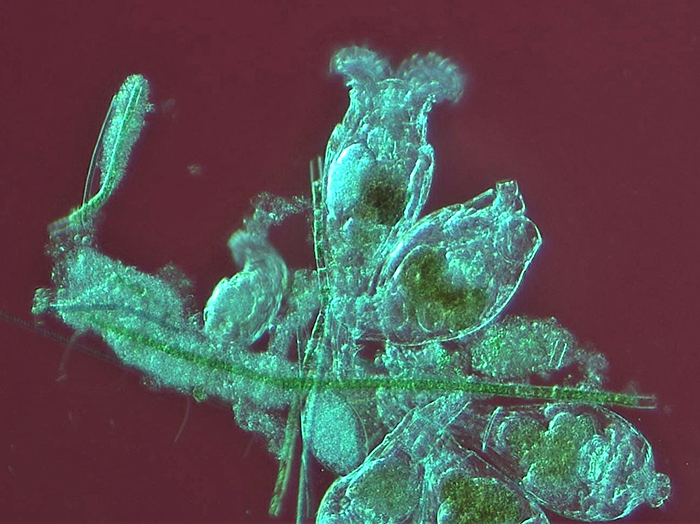 Alan Jones: Rotifer colony
Alan Jones: Rotifer colony
Sample taken from home aquarium for cold-water fish. Wet mount with coverslip.
Brunel SP500 trinocular, objective 20×, HDMI, USB camera.
Red ground achieved by placing a home-made central red stop on top of the condenser lens and beneath the flip-top lens.
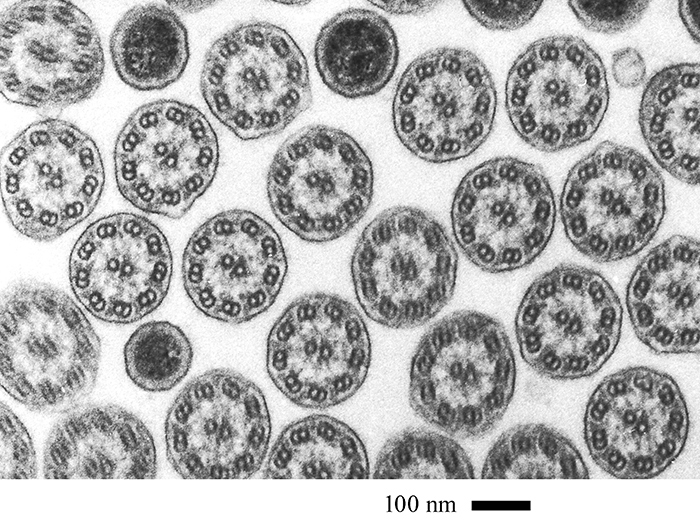 David Furness: Field of cilia (Barnard Award)
David Furness: Field of cilia (Barnard Award)
Field of cilia in transverse section obtained using my JEOL S100 transmission electron microscope. The project is about the structure and organisation of ciliates and is ongoing. The sample is a resin block made in 1981 containing rumen ciliates. These were sectioned at 90 nm thickness, stained with uranyl acetate and lead citrate, and examined in the TEM. Images were recorded onto Acros Neopan 100 35 mm film, developed and digitally scanned using a Canon flatbed scanner. The highly symmetrical cilia can be seen with their 9+2 arrangement of microtubules and associated proteins. In between are cylindrical bacteria (the dark profiles) also in transverse section that live between the animal’s cilia.
Processing the block took 3 days, cutting sections a week and staining 30 minutes. Observation time was 2–3 h.
 David Linstead: Dried flower head of Abutilon
David Linstead: Dried flower head of Abutilon
Dried dissected flower head of the Indian mallow Abutilon. Mounted dry on an opaque background. Victorian/Edwardian mount sold by Wheeler.
Imaged using incident light from a two-arm LED fibre optic diffused through parchment paper. Zeiss Jena ×10 apochromat objective on a Zeiss WL microscope. Canon EOS M3 camera afocally coupled to the trinocular tube.
A stack of 28 images processed with Zerene Stacker using the PMax method.
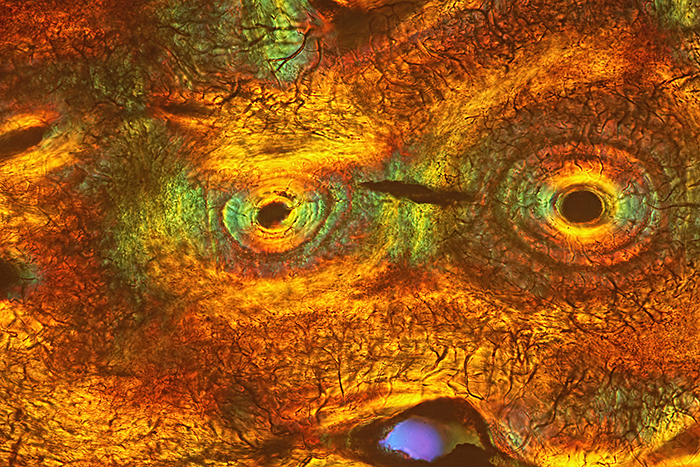 David Linstead: Section of fossilised shark tooth
David Linstead: Section of fossilised shark tooth
Thin transverse section of a fossil shark tooth. Slide mounted specimen from the Victorian/Edwardian era. Mounted and sold by C. M. Topping.
Imaged with a Nikon Diaphot inverted microscope. DIC illumination with a Nikon ×10 DIC objective and a Nikon variable retarder. Image not stacked or stitched.
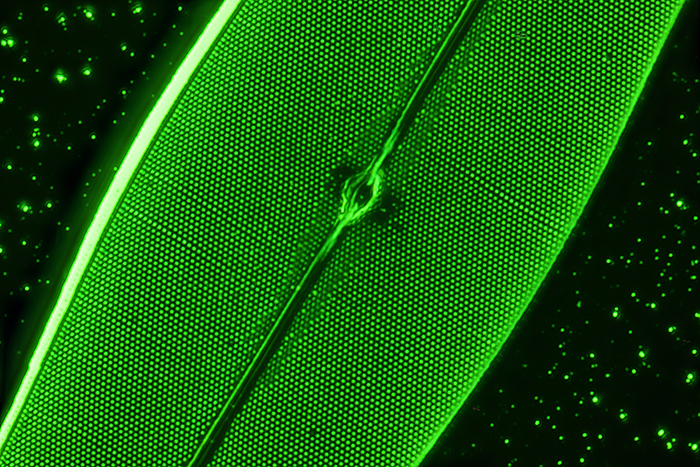 James Rider: Pleurosigma angulatum diatom
James Rider: Pleurosigma angulatum diatom
Aluminium-coated Pleurosigma angulatum diatom by John Dale. Reference: Balsam Post, July 1998, Issue No. 40, pp 12–14.
Microscope: Olympus BHS, 100× oil SPlanApo objective, 1.5× magnification changer, Aplanat Achromat condenser and 550 nm green filter.
Camera: Canon EOS 6D Mark II (full-frame), ISO 100, operated by home-built stacking system. Stack of 65 photographs using Affinity Photo 2.
Design and construction of stacking system took approximately 3 months. Development of the controlling software required a further 5 months.
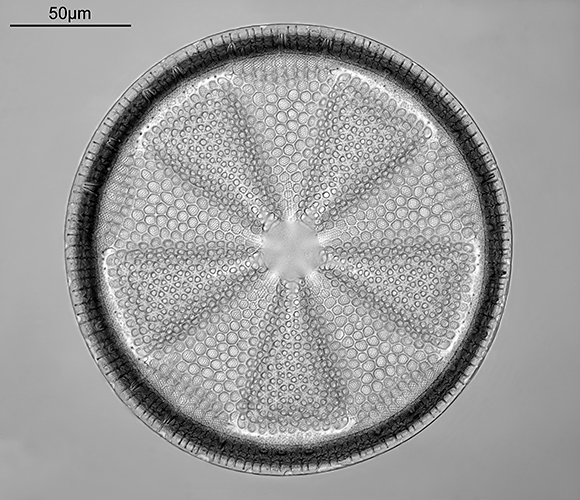 Jonathan Crowther: Actinoptychus heliopelta (Barnard Award)
Jonathan Crowther: Actinoptychus heliopelta (Barnard Award)
Eric Impey slide, Naphrax mountant, 1970.
Imaged on a modified Olympus BHB microscope using 450nm LED light.
Monochrome-converted Nikon D800 camera. 63× Leitz NA 1.4 Pl Apo objective, oil immersion. Olympus Aplanat Achromat condenser, oiled to slide and set to produce slightly oblique lighting. Nikon CF 5× photo eyepiece.
27 Images stacked using Zerene Stacker (DMap stacking). Final cropping, sharpening and tidying up of the image in Photoshop.
 Jonathan Crowther: Pleurosigma angulatum
Jonathan Crowther: Pleurosigma angulatum
Horace Dall slide of aluminium-coated diatom, 1981.
Imaged on modified Olympus BHB microscope using 365nm LED light.
Monochrome-converted Nikon D800 camera. 40× Nikon NA 1.4 UV-F objective, glycerine immersion. Olympus Abbe condenser, glycerine immersion, one side blocked underneath to produce oblique lighting. Nikon CF 5× photo eyepiece.
7 images stacked using Zerene Stacker (DMap stacking). Final cropping, sharpening and tidying up in Photoshop.
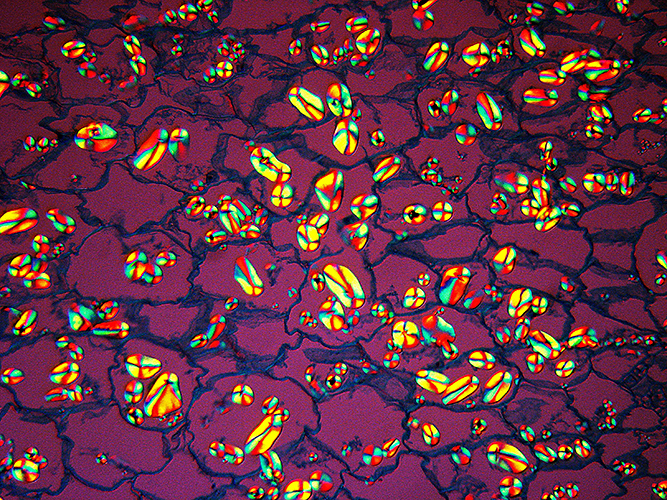 Mike Gibson: Potato starch viewed using polarised light
Mike Gibson: Potato starch viewed using polarised light
Photograph of a prepared Biosil slide by the late John Wells.
Taken with a Sony compact DSC W35 digital camera, shutter speed 1/15s at f/5.2, ISO 100.
Microscope technique: polarized light.
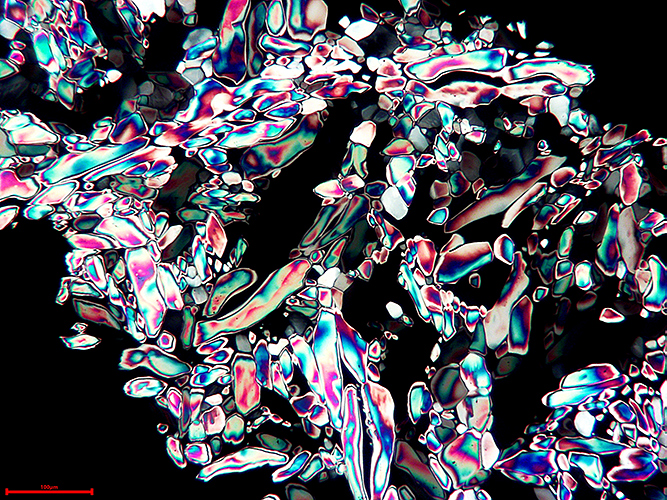 Mike Gibson: Urea crystals
Mike Gibson: Urea crystals
Urea crystals photographed from a prepared Biosil slide by the late John Wells.
Microscope technique: polarized light
Camera used: A USB 5 Megapixel eyepiece camera attached to a Wild M20 microscope and linked in tethered mode to a laptop computer.
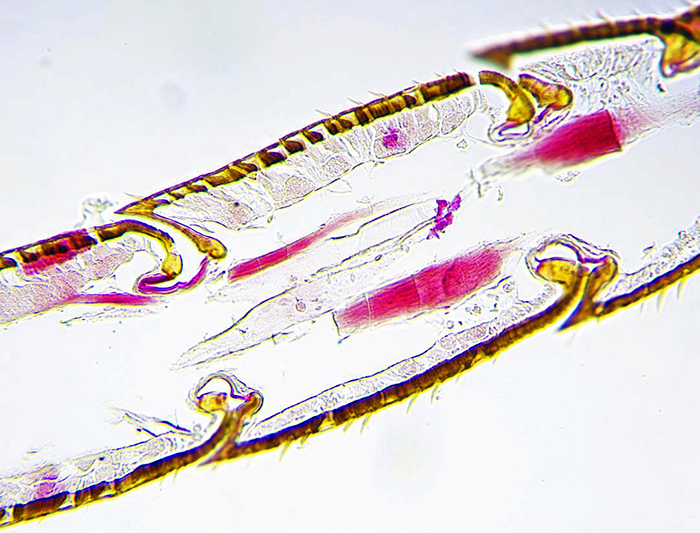 Mike Smith: L.S. honeybee antenna (Barnard Award)
Mike Smith: L.S. honeybee antenna (Barnard Award)
Longitudinal section of honeybee antenna from a slide of my own making.
×16 Objective on Leitz Orthoplan microscope, Swift 18MP camera.
Bright-field image, post capture processing by ToupVew software.
I had to section many bee heads, not always successfully, but did manage to capture this section. Stain was Cason’s, a one-step version of Mallory’s triple stain. The image shows the joint articulation and the lining of sensory cells that give the bee its extraordinary sense of “smell”.
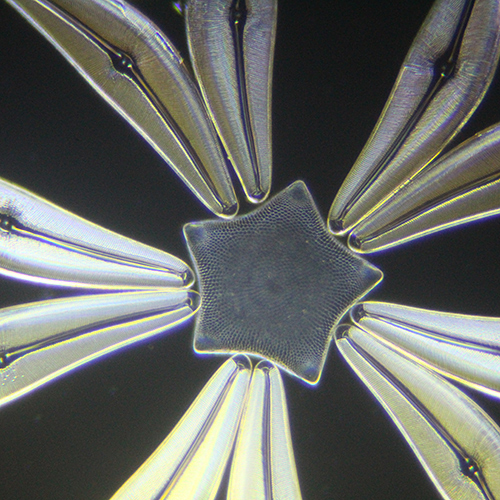 Nigel Williams: Klaus Kemp diatom star
Nigel Williams: Klaus Kemp diatom star
Detail from Klaus Kemp diatom star exhibition mount.
Dark-ground illumination using ×400 magnification on Wild M20 microscope.
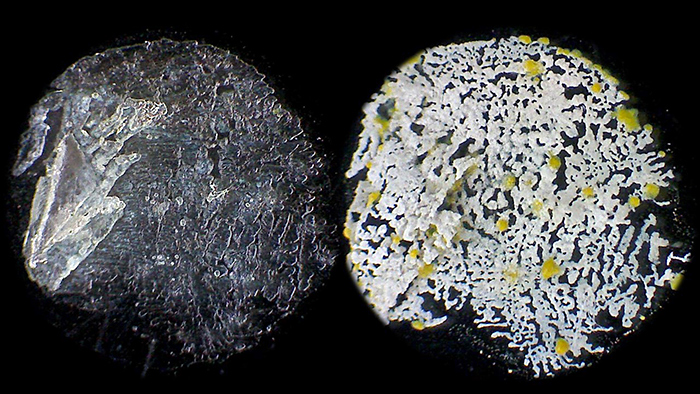 Pam Hamer: Copperas ore (Barnard Award)
Pam Hamer: Copperas ore (Barnard Award)
I collected a stone from a beach on the Isle of Wight and have been investigating it. I now believe it is a Copperas ore, pieces of which were collected from the Dorset coast many years ago and used as a mordant in the dyeing industry. The stone breaks down to form ferrous sulphate via the heptahydrate. This is deliquescent, absorbing water at high humidity and drying when the humidity is lower. A water extract from the stone was dried on a slide. The left image shows the deposit in high humidity. A month later the humidity was less and the deposit had become white with yellow inclusions which I take to be sulphur. These are images of a spot on the slide, approximately 5 mm across, without a mountant or cover slip. Natural light, low power stereo with Eyecam.
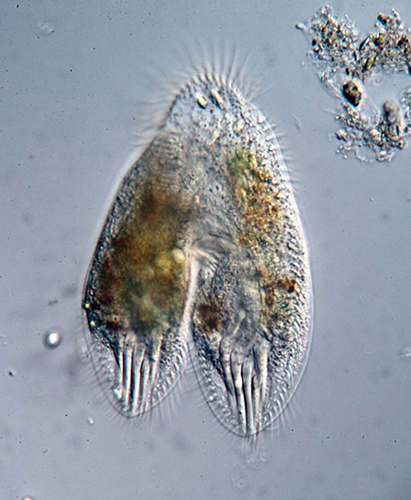 Thomas Consi: Hypotrich conjugating
Thomas Consi: Hypotrich conjugating
Image of a living hypotrich conjugating. Specimen collected from a local pond. Wet mount.
Microscope: Olympus BH-2, differential interference contrast (DIC).
Camera: Canon EOS 80D digital SLR.
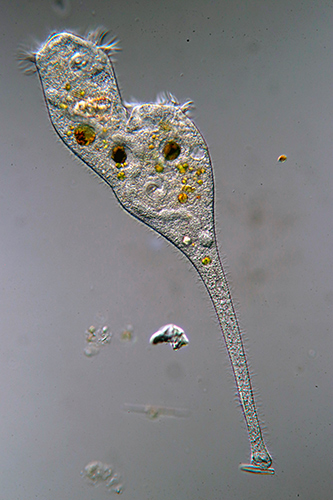 Thomas Consi: Stentor dividing (Barnard Award)
Thomas Consi: Stentor dividing (Barnard Award)
Image of a living Stentor dividing. Specimen collected from a local pond. Wet mount.
Microscope: Olympus BH-2, 20× objective, differential interference contrast (DIC)
Camera: Canon EOS 80D digital SLR.

